Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: In 2021, over 300,000 patients in the United States used adalimumab, making it one of the highest-grossing drugs globally. In 2023, patent exclusivity for the bio-originator form of adalimumab expired and eight biosimilars were introduced to the U.S. market. The arrival of these biosimilars has been eagerly awaited given the potential to reduce costs and improve access. Here we assess uptake of biosimilar adalimumab in the first nine months of 2023 overall, by patient characteristics and by practice.
Methods: Data derive from the Rheumatology Informatics System for Effectiveness (RISE) registry, a national EHR database from over 1100 US rheumatologists. We included 11,571 patients age > 18 years, with Medicare, Medicaid or private insurance, administered adalimumab (bio-originator or biosimilar) between Jan 2023 to Sept 2023. To evaluate new starts, we defined a subset of users with a visit in the 365 days before initiating use without a prescription for adalimumab. To assess switching, we considered a patient’s first (if any) change in prescription. We report the uptake of biosimilars overall and analyze the demographic and clinical factors associated with uptake using standardized mean differences. We assessed the distribution of uptake for each biosimilar product, the percent of new starts on a biosimilar, and the frequency of switching to a biosimilar. Among practices, we also calculated the median percent of patients prescribed a biosimilar.
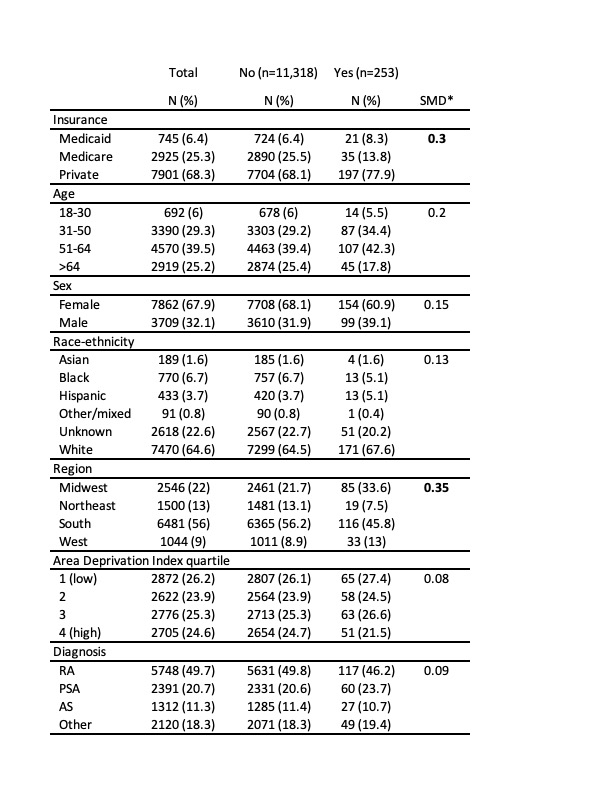
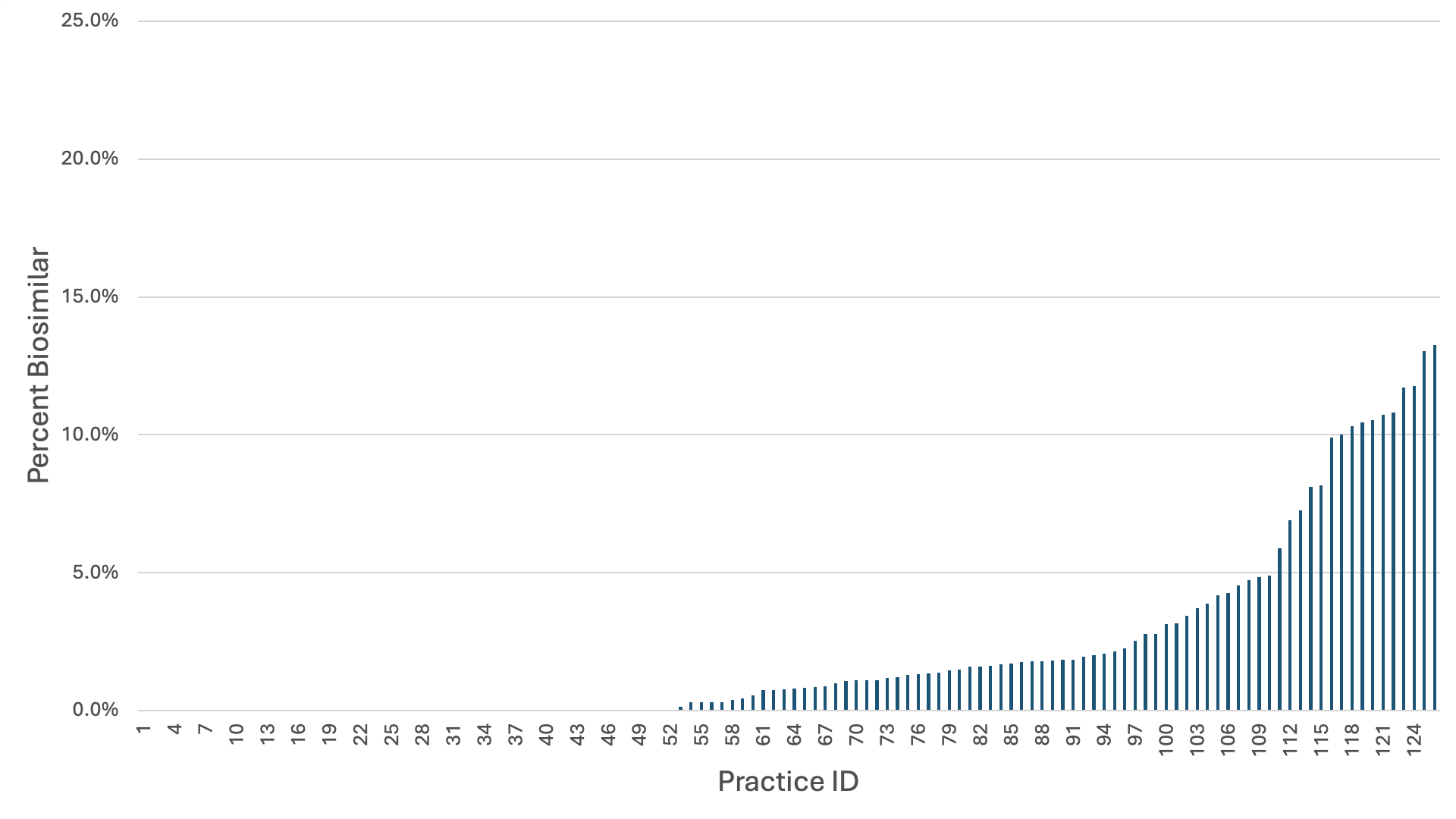
Results: In the first nine months of 2023, 253 of 11,571 (2.2%) patients received a prescription for a biosimilar version of adalimumab. There were only small differences in uptake by demographics or clinical characteristics with the largest differences by insurance and geographic region. Those with private insurance (2.5%) or Medicaid (2.8%) were more likely to use a biosimilar than those with Medicare (1.2%). Patients in the Midwest (3.3%) and West (3.2%) were more likely to receive a biosimilar than patients in the Northeast (1.3%) or South (1.8%) (Table 1). 70.4% (n=181) of biosimilar prescriptions were adalimumab-atto, which was the first released. Among new starts, 143 of 5133 (2.8%) were on a biosimilar. 96 patients switched from bio-originator adalimumab to a biosimilar and 14 patients switched from biosimilar to bio-originator. By practice (n=126), the median percent of patients receiving biosimilar adalimumab was 0.8% (IQR: 0 – 2.1%) with 10 practices having >10% of patients on biosimilar adalimumab (Figure 1).
Conclusion: We found critically low uptake of biosimilar adalimumab in the first nine months of 2023. Early indications are that formulary coverage has been uneven with a much higher percentage of formularies covering bio-originator adalimumab and no formularies have given a biosimilar a preferential tier or mandated step therapy. Delayed uptake of biosimilars means reduced savings for patients and tax payers, and discourages market entry for future biosimilars.
*SMDs <0.2 were considered as ‘very small’, 0.2-0.5 ‘small’, 0.5-0.8 ‘medium’ and >0.8 ‘large’.
Disclaimer: This data was supported by the ACR’s RISE Registry. However, the views expressed represent those of the authors, not necessarily those of the ACR.
To cite this abstract in AMA style:
Roberts E, Schmajuk G, Yazdany J. Very Low Uptake of Biosimilar Adalimumab in the First 9 Months of Availability in Rheumatology [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/very-low-uptake-of-biosimilar-adalimumab-in-the-first-9-months-of-availability-in-rheumatology/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/very-low-uptake-of-biosimilar-adalimumab-in-the-first-9-months-of-availability-in-rheumatology/