Session Information
Date: Sunday, November 8, 2015
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The BASDAI, BAS-G, and a VAS for spinal
pain (VAS-pain) are used to assess disease outcomes in AS. The intervals with
which these patient-reported outcome measures (PROMs) are assessed vary widely,
depending on the purpose of the assessment, and as such variability in outcome
may occur. However, it is currently unknown what the extent and relevance of this
variability is, and what the consequences of applying increasing intervals are on
follow-up of individual patients. The aim of the study was to evaluate in AS 1)
the variability of self-reported disease activity, patient global and spinal
pain during 2 years of follow up, 2) the clinical relevance of this variability
on a patient level, and 3) the effect of increasing intervals between two measurements
on this variability on a measurement level.
Methods: Dutch patients from the Outcome in AS International
Study (OASIS) completed the BASDAI, BAS-G, and VAS-pain every 2 months during 2
years. Mixed linear models were used to analyze time trends in the average
scores over time. The minimal clinically important difference (MCID) was used
to detect relevant changes (worsening or improvement) between two measurements. On a patient level, the frequency of exceeding the MCID, using
intervals of 2 months, was calculated. Next, the intervals between two measurements (from 2- to 24-months) were
increased to investigate the variability using all available measurements from
individual patients.
Results: 90 patients (mean age 47.3 (SD 11.4) years, 68% male, mean symptom duration 25.2 (SD
11.3) years) completed the PROMs. The mean of each measure was stable over time. On the patient level,
however, large variability was found. Using 2-months intervals, the average frequency of exceeding the MCID (in either direction) was 4.7 (SD 2.5) times for the BASDAI, 3.3
(SD 2.3) for the BAS-G, and 2.8 (SD 2.0) for the VAS-pain in the 2-year period
(from a maximum of 12). On an
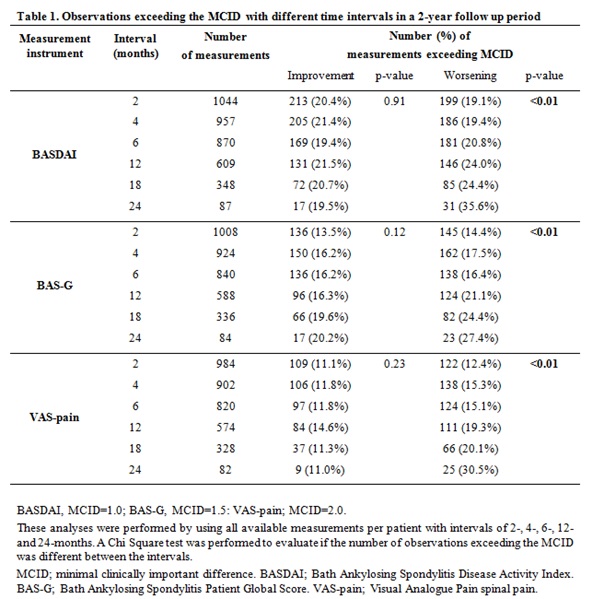
observational level, exceeding
of the MCID was frequently seen in all intervals. This markedly increased with
prolongation of the interval in observations showing worsening over time (Table
1).
Conclusion: Substantial variability in the BASDAI,
BAS-G, and VAS-pain was found in individuals over time. Clinically relevant
changes were frequently observed, especially in observations with worsening
more frequently relevant changes occurred with prolongation of the intervals.
This intermediate information between two measurements, which may be clinically
important, might be missed when intervals are prolonged.
To cite this abstract in AMA style:
Essers I, Busch M, Boonen A, Ramiro S, van der Heijde D, Landewé RBM, van Tubergen A. Variability over Time in Patient-Reported Outcome Measures in Patients with Ankylosing Spondylitis [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/variability-over-time-in-patient-reported-outcome-measures-in-patients-with-ankylosing-spondylitis/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/variability-over-time-in-patient-reported-outcome-measures-in-patients-with-ankylosing-spondylitis/