Session Information
Date: Sunday, November 7, 2021
Title: Measures & Measurement of Healthcare Quality Poster (0623–0659)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: The RAPID3 is a patient-reported pooled index of three measures: function, pain, and patient global estimate of status, that is used to classify disease severity1. It was developed in rheumatoid arthritis, but multiple studies have shown that it is useful in many rheumatic diseases, including Psoriatic Arthritis (PsA)2,3. We assessed the correlation of RAPID3 with other standard scores used in PsA in a private clinical practice setting.
Methods: Adult patients with a diagnosis of PsA seen between February 2012 and November 2020 were retrospectively identified. We collected data including demographics, clinical measures and 4 disease activity measures: RAPID3,DAPSA, cDAPSA and CDAI from random routine care visits.
Spearman’s correlations were calculated between RAPID3 and the other scores. Cross-test consistency of disease activity (remission, low, moderate, high) between different scores using kappa agreement analysis was calculated. Using ROC, we assessed the values of RAPID3 which best correlated with these levels of consistency. All calculations were performed using Stata Software v14 (Plano TX). IRB approval was obtained.
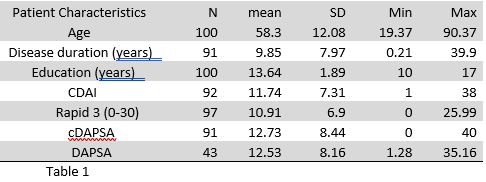
Results: The study included 100 patients with PsA. Patients’ characteristics and scores are presented in table 1.
Spearman correlation analysis demonstrated a very strong correlation between RAPID3 and both DAPSA and cDAPSA (r=0.86) as well as a strong correlation between RAPID3 and CDAI (r=0.77).
There was strong agreement between RAPID3 remission score and DAPSA and cDAPSA remission scores, Kappa: 0.78 (p < 0.0001) and 0.71 (p < 0.0001) respectively.
The level of agreement dropped when we combined remission and low activity scores together, Kappa 0.56 (p < 0.0001) and 0.55 (p < 0.0001) respectively, although this remained in the moderate range.
Based on the ROC analysis, we redefined the RAPID3 score categories. A score of ≤3 was considered remission. >3 and ≤11 was considered low disease activity. >11 and ≤15 was considered moderate disease activity and >15 was considered high disease activity. Based on these categories, the level of agreement between RAPID3 and DAPSA and cDAPSA, when combining remission and low activity scores, significantly increased, Kappa 0.72 (p< 0.0001) and 0.71 (p < 0.0001) respectively.
Conclusion: This study supports the hypothesis that RAPID3 is as an effective disease activity measure in PsA. Our results show a strong to very strong correlation between RAPID3 and other standard scores such as DAPSA and cDAPSA used as a quantitative assessment of PsA disease activity at baseline and to measure treatment response. The advantage of RAPID3 compared to other disease activity measures is that it is a simple score that can be filled out entirely by the patients with minimal time or effort from the rheumatologist1,2,3.
We suggest that redefining the RAPID3 score categories to a score < 11 for low disease activity and >15 for high disease activity, will categorize disease activity more accurately.
Ref
1- Pincus, T, et al. The Journal of Rheumatology 35.11 (2008): 2136-2147.
2- Pincus, T et al. Bulletin of the NYU. 70 Suppl 1 (2012): 30-6.
3- Coates, LC et al. Arthritis Care & Research. 70,8 (2018): 1198-1205.
To cite this abstract in AMA style:
Fares J, Healy A, Bergman M. Value of the Routine Assessment of Patient Index Data 3 (RAPID3) as a Disease Activity Measure in Patients with Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/value-of-the-routine-assessment-of-patient-index-data-3-rapid3-as-a-disease-activity-measure-in-patients-with-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/value-of-the-routine-assessment-of-patient-index-data-3-rapid3-as-a-disease-activity-measure-in-patients-with-psoriatic-arthritis/