Session Information
Date: Monday, October 22, 2018
Title: Spondyloarthritis Including Psoriatic Arthritis – Clinical Poster II: Clinical/Epidemiology Studies
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The Patient-Reported Outcomes Measurement Information System (PROMIS) developed by the US National Institutes of Health is a patient-reported outcomes system designed to measure disease burden across a wide array of chronic diseases and the general population1. We sought to evaluate the validity of selected PROMIS measures in Ankylosing Spondylitis (AS) patients across self-reported Assessment in Spondyloarthritis International Society core set and patient-identified domains.
Methods: Patients at the Houston, Texas site of the Prospective Study of Outcomes in Ankylosing Spondylitis, a longitudinal, prospective, AS cohort from Sept 2017-May 2018 were enrolled in a sub-study in which they completed PROMIS short forms (SFs) assessing global health, depression, fatigue, pain and physical function in addition to the other legacy measures collected in the cohort study. PROMIS SFs ranged from 3-12 questions. We assessed internal consistency using Cronbach’s alpha. Content validity was assessed by asking patients if the PROMIS SF questions related to their disease. We assessed construct validity through examination of score distributions, floor effects and through examination of the Spearman’s correlation coefficients between PROMIS measures and existing legacy AS measures (e.g. BASDAI, BASFI, Global Numeric Rating Scale (NRS), Pain NRS and CES-D) of similar domains. We hypothesized that there would be moderate to strong correlation (e.g., 0.6-1) between the PROMIS measures and the target legacy measures.
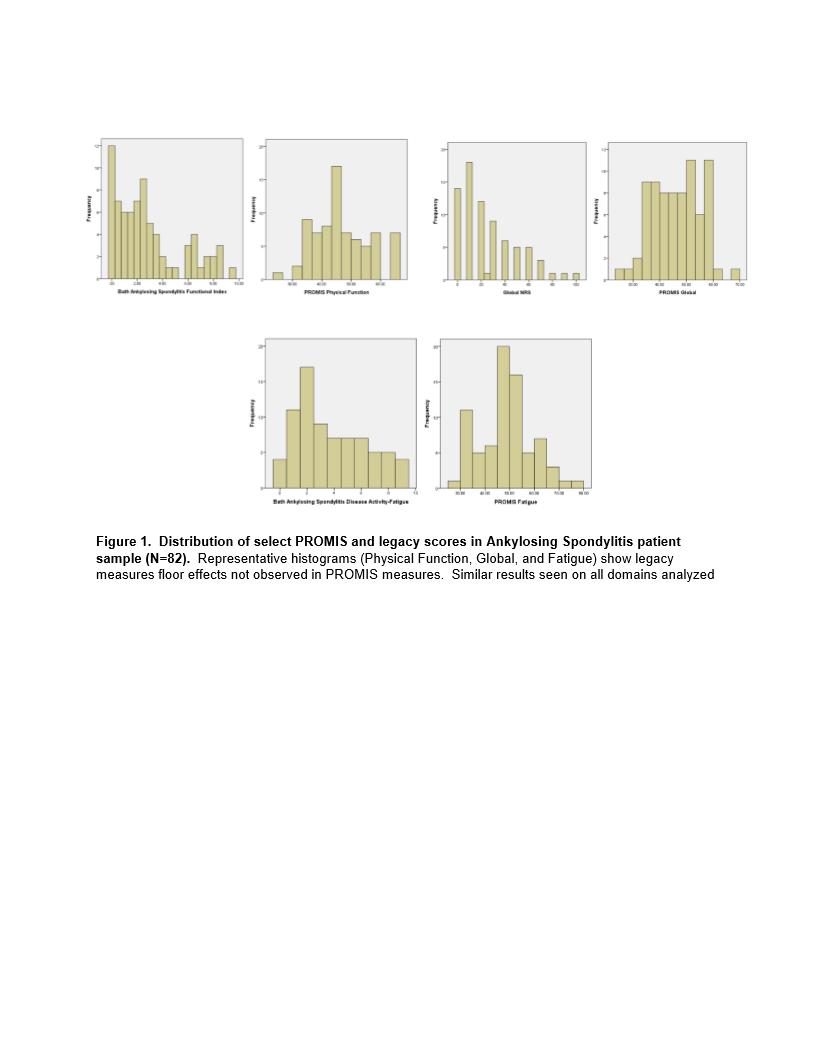
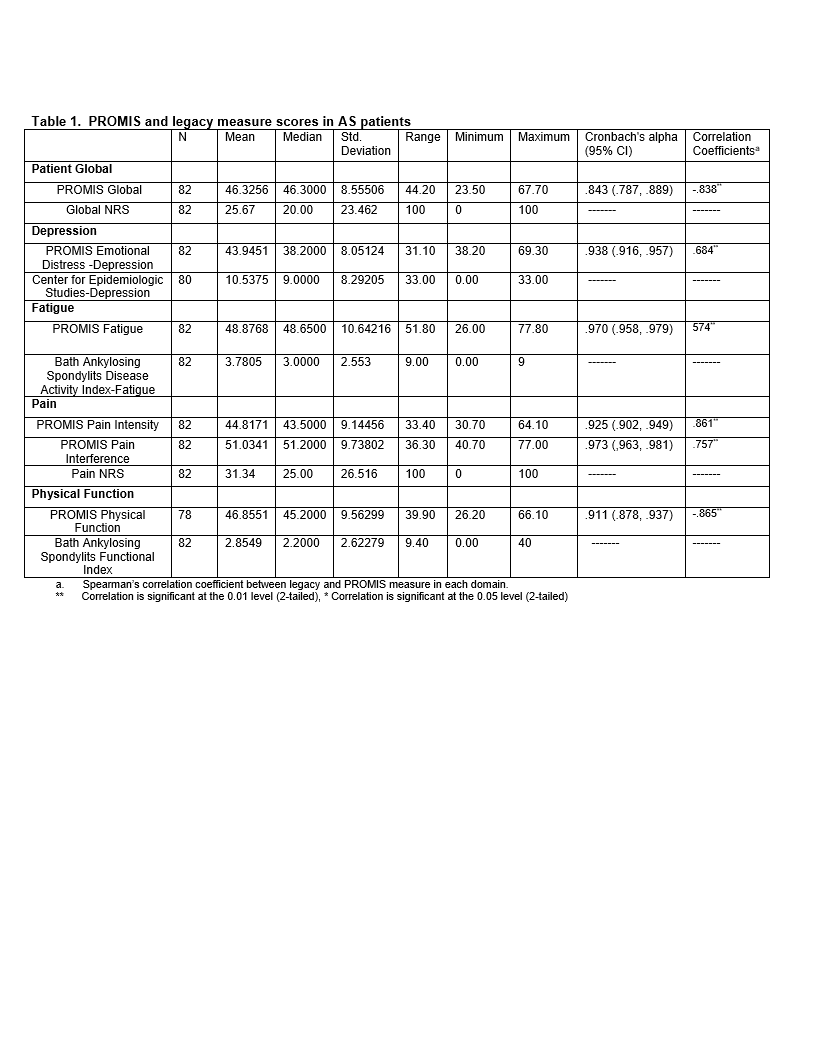
Results: Participants (n=82) were mostly male (75%), white (84%), college educated (82%) with a mean age of 53 years. All patients felt the PROMIS SFs addressed AS disease aspects. Legacy measures demonstrated a floor effect that was not present in the PROMIS SFs (Figure 1). Strong internal consistency was noted in the PROMIS SFs ranging from .843-.973. PROMIS Global, Depression, Fatigue, Pain, Physical Function correlated moderately-strongly (rho .684-.865) with the appropriate legacy measures (Table 1). Patients reported time to complete the entire PROMIS SFs packet was <10 minutes overall.
Conclusion: This study demonstrates the content and construct validity of PROMIS SFs to assess AS symptoms from a single-center sample of AS patients. Further research is needed to assess discrimination (the ability of PROMIS SF to distinguish disease activity groups), responsiveness, feasibility/resource burden, and translations for AS patients.
1. HealthMeasures. Intro to PROMIS [Internet. Accessed June 4, 2018.] Available from: www.healthmeasures.net
To cite this abstract in AMA style:
Hwang M, Ogdie A, Reveille JD. Validity of Patient-Reported Outcomes Measurement Information System Measures in Ankylosing Spondylitis Patients [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/validity-of-patient-reported-outcomes-measurement-information-system-measures-in-ankylosing-spondylitis-patients/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validity-of-patient-reported-outcomes-measurement-information-system-measures-in-ankylosing-spondylitis-patients/