Session Information
Date: Sunday, November 13, 2016
Title: Fibromyalgia, Soft Tissue Disorders, Regional and Specific Clinical Pain Syndromes
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: The provisional criteria of the American College of Rheumatology (ACR) 2010 and the 2011 self-report modification for survey and clinical research are widely used for fibromyalgia diagnosis. To determine the validity, usefulness, potential problems and modifications required for the criteria, we assessed multiple research reports published in 2010-2015 in order to provide a 2016 update to the criteria.
Methods: We reviewed 14 validation studies that compared 2010/2011 criteria with ACR 1990 gold standard classification and clinical criteria, as well as epidemiology, clinical and databank studies that addressed important criteria-level variables, including the polysymptomatic distress (PSD) scale – also known as the fibromyalgia severity (FS) scale. Based on definitional differences between 1990 and 2010/2011 criteria, we interpreted 85% sensitivity and 90% specificity as excellent agreement.
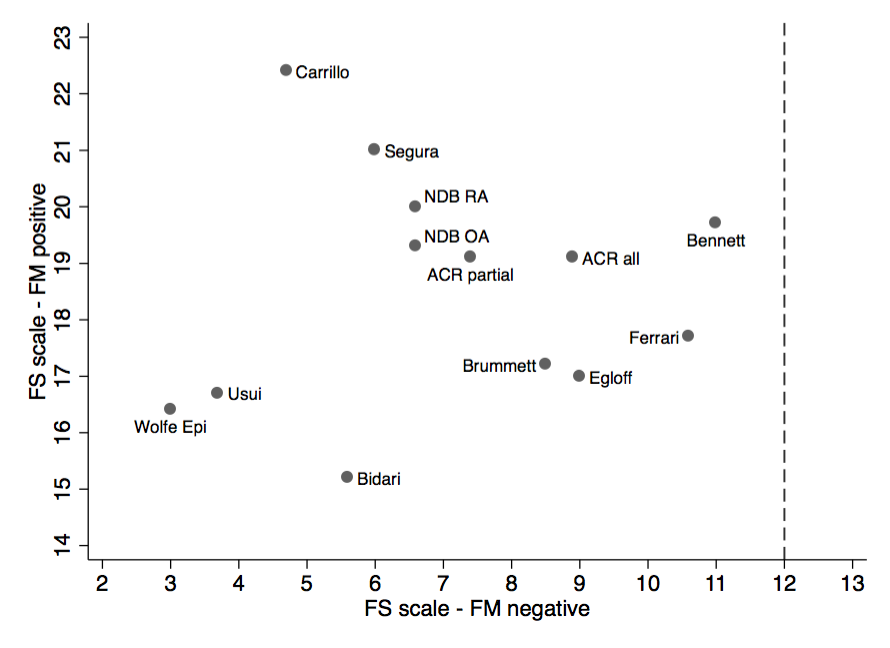
Results: Against 1990 and clinical criteria, the median sensitivity and specificity of the 2010/2011 criteria was 86% and 90% (Figure 1). There was a strong association between ACR 1990 tender point levels in cases and controls, and among 2010/2011 PSD/FS scale results (Figure 2). Data from the ACR 2010 study indicated the correlation between tender points and PSD/FS scale was 0.781. Plotting the levels of PSD in FM (+) and FM (-) cases (Figure 3) provided further insight into the effect of symptom severity on diagnosis. Fibromyalgia is milder in population studies. Studies of fibromyalgia criteria are sensitive to selection issues and severity, and to characteristics of control subjects. Various study authors were uncertain as to how to interpret criteria in the presence of other medical disorders. 2010/2011 criteria led to misclassification when applied to regional pain syndromes, but when a test modified widespread pain criterion was added misclassification was eliminated.
Conclusion: The fibromyalgia criteria have good sensitivity and specificity, and were perceived as useful and easy to use. Results of these analyses will be incorporated into revised criteria to eliminate observed problems. 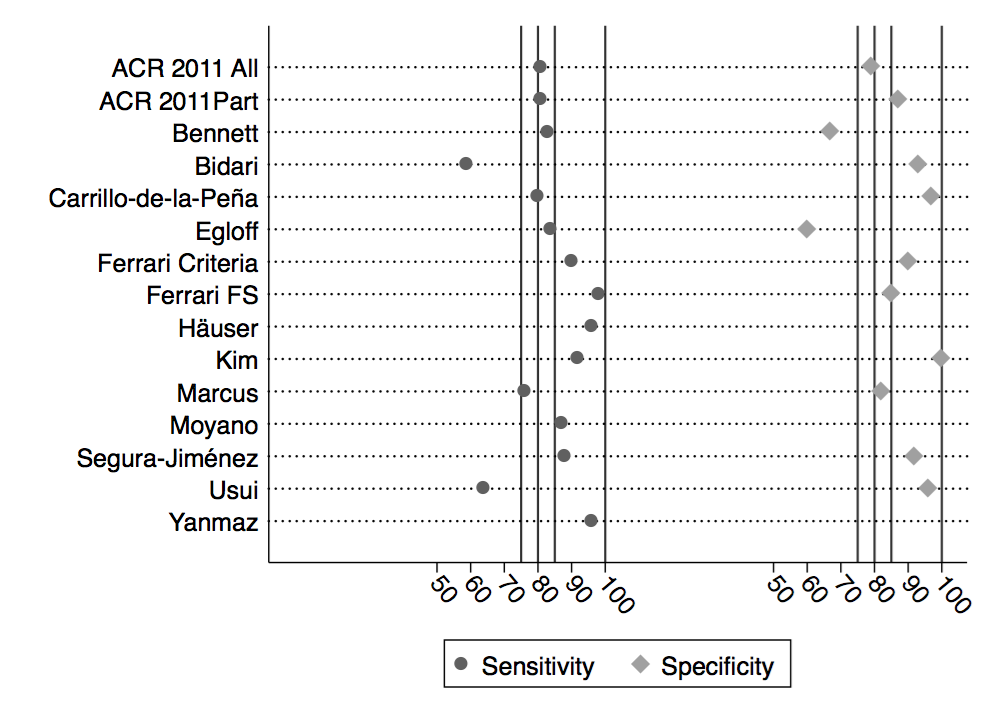
To cite this abstract in AMA style:
Wolfe F, Clauw DJ, FitzCharles M, Goldenerberg D, Häuser W, Katz RS, Mease PJ, Russell A, Russell IJ, Walitt B. Validation Studies of the American College of Rheumatology (ACR) 2010 Fibromyalgia Diagnostic Criteria and the 2011 Self-Report Modification for Survey and Clinical Research [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/validation-studies-of-the-american-college-of-rheumatology-acr-2010-fibromyalgia-diagnostic-criteria-and-the-2011-self-report-modification-for-survey-and-clinical-research/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-studies-of-the-american-college-of-rheumatology-acr-2010-fibromyalgia-diagnostic-criteria-and-the-2011-self-report-modification-for-survey-and-clinical-research/