Session Information
Date: Sunday, November 8, 2015
Title: Systemic Lupus Erythematosus - Clinical Aspects and Treatment Poster Session I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
Disease Specific Patient reported outcome measure tools
capture unique domains relevant to patients with a specific disease. LupusPRO
is targeted towards measuring health (HRQOL) and non-health related quality of
life (Non HRQOL) among patients with systemic lupus erythematosus (SLE).
LupusPRO V 1.7 and its translated versions in various languages have
measurement equivalence and are responsive to changes in changes in patient
reported changes in health and physician based disease activity assessments.
However, items pertaining to sleep, or impact of vitality and pain on quality
of life were placed together in the same domain (Vitality-Pain) based on factor
loadings in initial studies and in interest of parsimony. For purposes of charting
changes in individual components of this combined domain (Pain-Vitality), we
separated the sleep, pain and vitality items into separate domains, keeping
intact the concepts, content and language of the items, in version 1.8. We
present the psychometric properties of LupusPRO v1.8, with special attention to
LupusPRO domains of sleep, vitality and pain.
Methods:
Fifty consenting SLE patients fulfilling ACR classification
criteria were given self-administered surveys (MOS SF36 FACIT-Fatigue, Pain, Insomnia
Severity Index for sleep, Perceived Stress Scale (PSS)-4, Patient Health
Questionnaire-9 (PHQ-9) for depression, LupusPRO V 1.8) to complete at routine
care visit. Disease activity and damage were assessed at visit using SELENA-SLEDAI
(SS), numeric BILAG and SLICC-SDI/ACR (SDI). Internal consistency reliability
(ICR) for each domain was obtained using cronbach alpha. Convergent construct validity
(CV) with corresponding domains of SF36 was tested using spearman correlation
coefficient.
Results:
Mean (SD) age was 41.4±13 yrs., 90% participants
were women. Ethnic background was as follows: 56% Blacks, 24% Whites, 10%
Asians and 10% others. Median (IQR) values of PGA, total SS, and SDI were 0.5
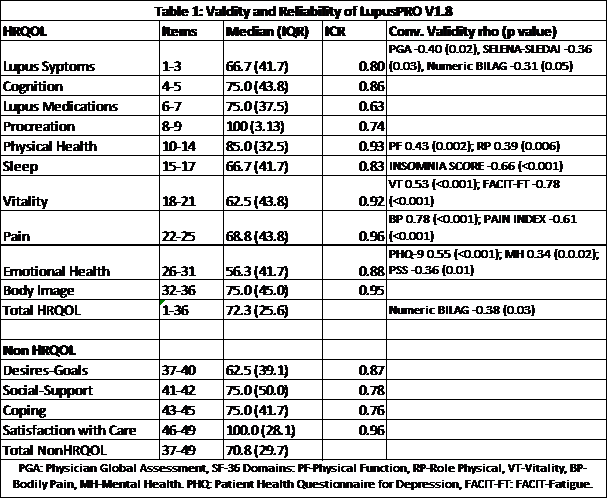
(0.8), 4.0(6.0) and 1.0(1.0), respectively. Results for LupusPRO V1.8 domains
descriptives, ICR and CV are shown in Table 1. ICR for LupusPRO sleep, vitality
and pain domains exceeded 0.80. LupusPRO Sleep domain scores inversely and
strongly correlated with the Insomnia severity index scores, while LupusPRO Vitality
(4 items) correlated strongly with FACIT-Fatigue (13 items) and SF36 Vitality
scores. LupusPRO Pain domain correlated strongly with the Pain score and SF36
Bodily pain domain. Lupus symptom domain (3 items) showed significant
correlation with PGA, SS and BILAG. LupusPRO domains of Physical and Emotional
Health had good ICR and significant correlation with corresponding SF36 domains
(Table 1).
Conclusion:
LupusPRO V1.8 (including its sleep,
vitality and pain domains) has acceptable reliability and validity. It offers
significant advantages over LupusPRO V1.7 and its 36 items comprehensively
cover HRQOL in SLE.
To cite this abstract in AMA style:
Jolly M, Gandhi N, Nevares A, Sequeira W. Validation of Lupuspro V1.8, Disease Targeted Patient Reported Outcome for Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/validation-of-lupuspro-v1-8-disease-targeted-patient-reported-outcome-for-systemic-lupus-erythematosus/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-lupuspro-v1-8-disease-targeted-patient-reported-outcome-for-systemic-lupus-erythematosus/