Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Childhood-onset systemic lupus erythematosus (cSLE) is a multisystem autoimmune disease that is characterized by episodes of flares followed by improvement in response to therapy. To assess the clinically relevant response and the efficiency of therapies in cSLE, sensitive and well-correlated criteria with changes in improvement are needed.
Objective: To prospectively validate the PRINTO/ACR Criteria of Response to Therapy (PCI) & assess their ability to capture clinically relevant improvement (CRI).
Methods: Consensus formation methodology (Delphi, nominal group technique; 77 % consensus level) was used to newly define CRI. Using prospective cSLE data, patient profiles (PPs) were developed which provided information at a baseline & follow-up visit, including the cSLE core response variables [CRVs: SLEDAI, the urinary protein-creatinine ratio (PCR), physician global assessments of cSLE disease activity, patient global assessment of well-being, quality of life score]. Twelve experts (>10 yrs experience managing cSLE) were asked to rate disease courses of up to 200 PPs using 2 scales: (1) no change/worsening, minor, moderate, or major improvement; and (2) clinically relevant improved, yes/no. Each PP was determined of the “true” course based upon the majority opinion of the raters. Multinomial logistic regression models (MLRs) were used to predict the true course and develop algorithms to calculate the continuous “improvement score” using absolute or percentage changes of the CRVs. ROC curves were analyzed for the MLR algorithms and AUC, specificity, sensitivity, and kappa statistics were calculated to assess the improvement scores for accuracy. Results were reviewed by the expert panel for further discussion and recommendation.
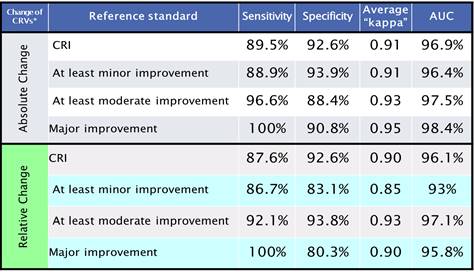
Results: The consensus definition of CRI was: “A clinically relevant improvement has occurred in a child with lupus if there are reduced signs of disease from active lupus. Although there may not be an improvement of lupus activity in all organ systems, there cannot be increased lupus activity in major organ systems (neuropsychiatric, hematological, gastrointestinal, renal, ophthalmological, and cardiopulmonary). Patient symptoms must be at least stable, and immunosuppressive therapy should be unchanged or decreased“ (100 % consensus). All 4 top PCI were highly sensitive and moderately accurate but specificity was lacking to various degrees of improvement or CRI. The MLR-based algorithms appeared more accurate than the PCI for CRI and minor/moderate/major improvement (Table 1).
Conclusion: The PCI are moderately accurate in this validation data set, but the MLR-derived criteria considering absolute CRV changes perform better in capturing CRI and cSLE improvement in general. If confirmed in larger validation studies, MLR-based algorithms may allow for a more effective assessment of response to therapy in cSLE.
Table 1. Multivariate Models for Improvement Criteria
To cite this abstract in AMA style:
Avar Aydin PO, Holland MJ, Appenzeller S, Ardoin SP, Avcin T, Beresford MW, Feldman BM, Flores F, Klein-Gitelman MS, Goilav B, Khubchandani R, Levy DM, Ravelli A, Ruperto N, Silva CA, Wenderfer SE, Ying J, Brunner HI. Validation of Clinically Relevant Improvement in Children and Adolescents with cSLE [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/validation-of-clinically-relevant-improvement-in-children-and-adolescents-with-csle/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-clinically-relevant-improvement-in-children-and-adolescents-with-csle/