Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: While the psoriasis area and severity index (PASI) is the most commonly used assessment tool for psoriasis severity, it has been criticized for its subjectivity and complexity. Expert training is needed to minimize errors, as well as inter-rater and intra-rater variability. It also burdens the provider and does not incorporate the patient’s perspective. We validate a simplified two-item palmPASI against previously established standardized measures of psoriasis involvement.
Methods: Adult patients (18 years or older) with psoriasis or psoriatic arthritis (PsA) in the Veterans Affairs Program to Understand the Longterm outcomes in Spondylo-ARthritis (PULSAR) registry participated in this study. Subjects complete a two item “palmPASI” questionnaire, using a 10-point scale to describe how psoriasis affected the patient and the degree of surface area involvement. These palmPASI scores were then compared to formal four-component PASI scores (erythema, induration, scaling, and involved area of lesions), as determined by health care personnel, as well as the Psoriasis Symptom Inventory (PSI) and Dermatology Life Quality Index (DLQI). Correlations were analyzed using Spearman correlations. The relationship between clinical feature and palmPASI scores was analyzed using logistic regression.
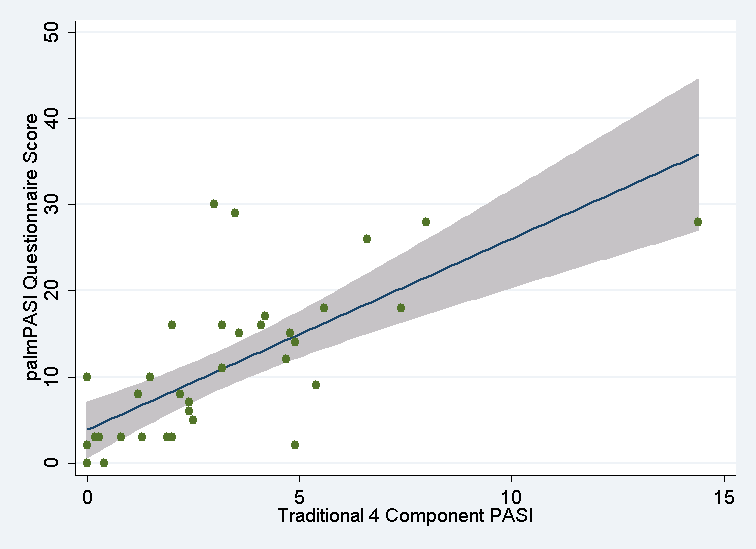
Results: A total of 352 subjects completed the palmPASI and a convenience sample of 40 were examined with the formal PASI. Strong correlations exists between palmPASI and PASI (Spearman’s rho=0.75, p<0.001; see Figure 1), PSI (Spearman’s rho=0.73, p<0.001), and DLQI (Spearman’s rho=0.77, p<0.001). Psoriasis area score was highly correlated with the four-component PASI (Spearman’s rho=0.75, p<0.006). The test-retest reliability of the palmPASI was excellent (rho=0.93, p<0.001). African-Americans and those with elevated c-reactive proteins demonstrated higher palmPASI scores.
Conclusion: Assessment of psoriasis and psoriatic arthritis disease activity is crucial when determining treatment efficacy in clinical research. The palmPASI incorporates patient perspectives, requires less than 10 seconds to score, and demonstrates validity when compared against standard psoriasis instruments. This instrument could potentially serve as a reliable and simple standardized tool to guide evidence-based management.
To cite this abstract in AMA style:
Mounessa J, Lynn D, Walsh J, Hashim M, Duong R, Dellavalle R, Caplan L. Validation of a Two-Question Patient Reported Outcome Measure for Psoriasis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/validation-of-a-two-question-patient-reported-outcome-measure-for-psoriasis/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-a-two-question-patient-reported-outcome-measure-for-psoriasis/