Session Information
Date: Saturday, November 12, 2022
Title: Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: We assess the effectiveness of COVID-19 vaccination in preventing SARS-CoV2 infection and COVID-19 hospitalizations in patients with psoriatic disease (PsD) and non-psoriatic controls, and the association of immune-modulating medications on these COVID-19-related outcomes.
Methods: A population-based study was performed using the data from the Clalit Health Services in Israel. We assembled a cohort of patients with diagnoses of psoriasis and/or psoriatic arthritis (collectively termed PsD) and matched them by age, sex, and clinic (1:5 ratio) with non-psoriatic controls. For assessment of vaccine effectiveness, the following 2 periods were analyzed separately: Period 1 which corresponds to initiation of COVID-19 vaccinations until initiation of booster vaccines (December 2020-August 2021); Period 2 corresponds to initiation of booster vaccines to end of study (August 2021-December 2021). Study outcomes included: SARS-CoV2 PCR positivity and hospitalization for COVID-19. For assessment of the association of medications and COVID-19-related outcomes, we performed a matched nested case-control study within the PsD cohort in which each case (SARS-CoV2 infection and COVID-19 hospitalization) was matched with 10 controls (negative for these outcomes). Vaccine effectiveness was assessed using regression models with time varying covariates for vaccination status adjusted for demographics, comorbidities and medication use.
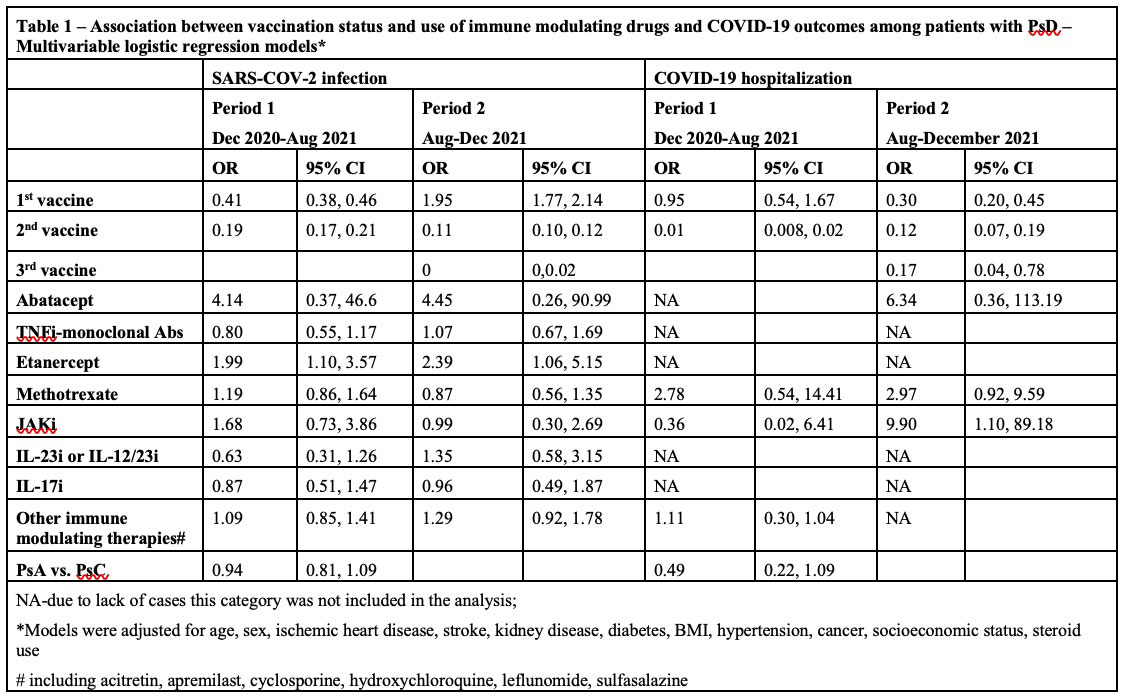
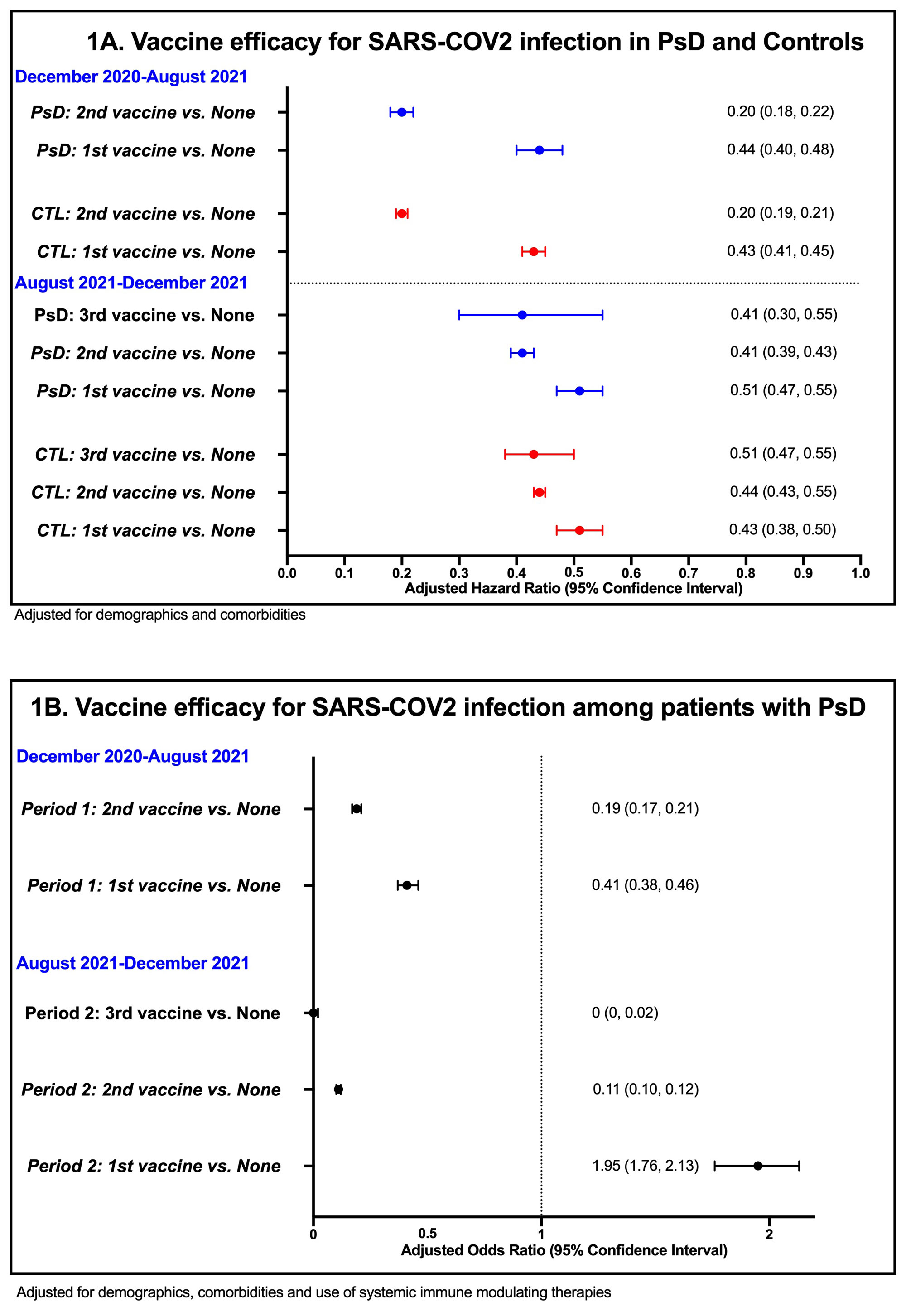
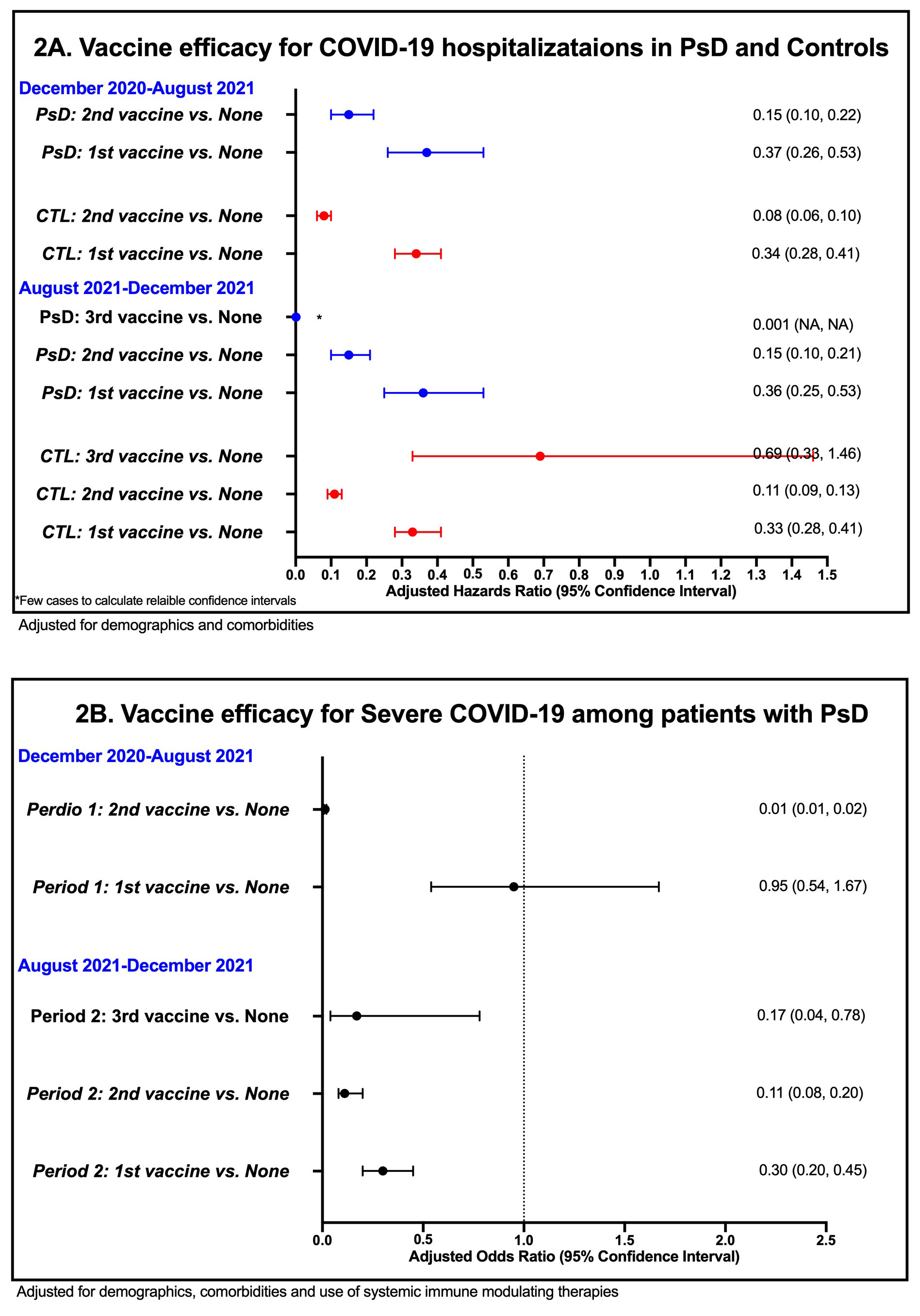
Results: A total of 128,754 patients with PsD and 600,439 controls (5,934 vs. 26,292 positive SARS-CoV2; 315 vs. 1,127 hospitalized, respectively). Vaccine effectiveness for SARS-CoV2 infection was similar among patients with PsD and controls (HR for 2nd vaccine: 0.20 vs. 0.20, respectively; Figure 1A). Vaccine effectiveness for COVID-19 hospitalization was also similar among PsD patients and controls (HR for 2nd vaccine: 0.15 vs. 0.08, respectively; Figure 2A). Booster vaccines remained effective in reducing risk of infections (HRs for of 3rdvaccine: 0.41) and hospitalization (HR for of 3rd vaccine < 0.01) among patients with PsD. When the analysis was restricted to patients with PsD and adjusted for use of systemic therapies, vaccines remained effective for SARS-CoV2 infections (OR period 1: 2nd vaccine: 0.19; OR period 2: 3rd vaccine: < 0.01; Figure 1B) and hospitalizations (OR period 1: 2nd vaccine: 0.01; OR period 2: 3rd vaccine: 0.17; Figure 2B). Use of etanercept was associated with higher risk of SARS-CoV2 infection and JAK inhibitors use was associated with higher risk of hospitalizations (Table 1). PsA status was not associated with higher risk of both outcomes compared to patients with psoriasis alone.
Conclusion: The COVID-19 vaccine has similar effectiveness in patients with PsD to that seen in non-psoriatic controls. Risk of COVID-19 hospitalization among PsD patients may be influenced by certain immune modulating therapies.
To cite this abstract in AMA style:
Eder L, Lavi I, Haddad A, Stein N, feldhamer I, DOV COHEN A, Saliba W, Zisman D. Vaccine Effectiveness Among Patients with Psoriatic Disease: A Population-based Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/vaccine-effectiveness-among-patients-with-psoriatic-disease-a-population-based-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/vaccine-effectiveness-among-patients-with-psoriatic-disease-a-population-based-study/