Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Uveitis is one of the most frequently reported extra-musculoskeletal manifestations of axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA).1,2 The prevalence of anterior uveitis is 23–33% and 2–25% in patients with axSpA and PsA, respectively.3,4 Secukinumab is a fully human monoclonal antibody that directly inhibits interleukin-17A and is approved for the treatment of axSpA and PsA. We previously reported that the incidence rate of uveitis was 1.4 per 100 patient-years in secukinumab-treated axSpA patients.1 The incidence rate of uveitis in patients with axSpA and PsA during placebo-controlled phase with secukinumab and placebo is reported here.
Methods: This post hoc analysis included comparative phase data from two distinct pools: axSpA cohort (PREVENT, MEASURE 1–5) and PsA cohort (FUTURE 1–5) of patients who received either 150 or 300 mg of secukinumab therapy or placebo. The placebo-controlled phase lasted up to Week 16 visit for all studies except PREVENT, where Week 20 was used. Patients who received secukinumab 75 mg (unapproved dose) were excluded from the analysis. Data on demographics, relevant or current medical conditions, prior use of number of tumor necrosis factor (TNF) alpha inhibitors, and concomitant treatment and doses were collected at baseline. The incidence of uveitis is reported as exposure-adjusted incidence rate per 100 patient-years of treatment.
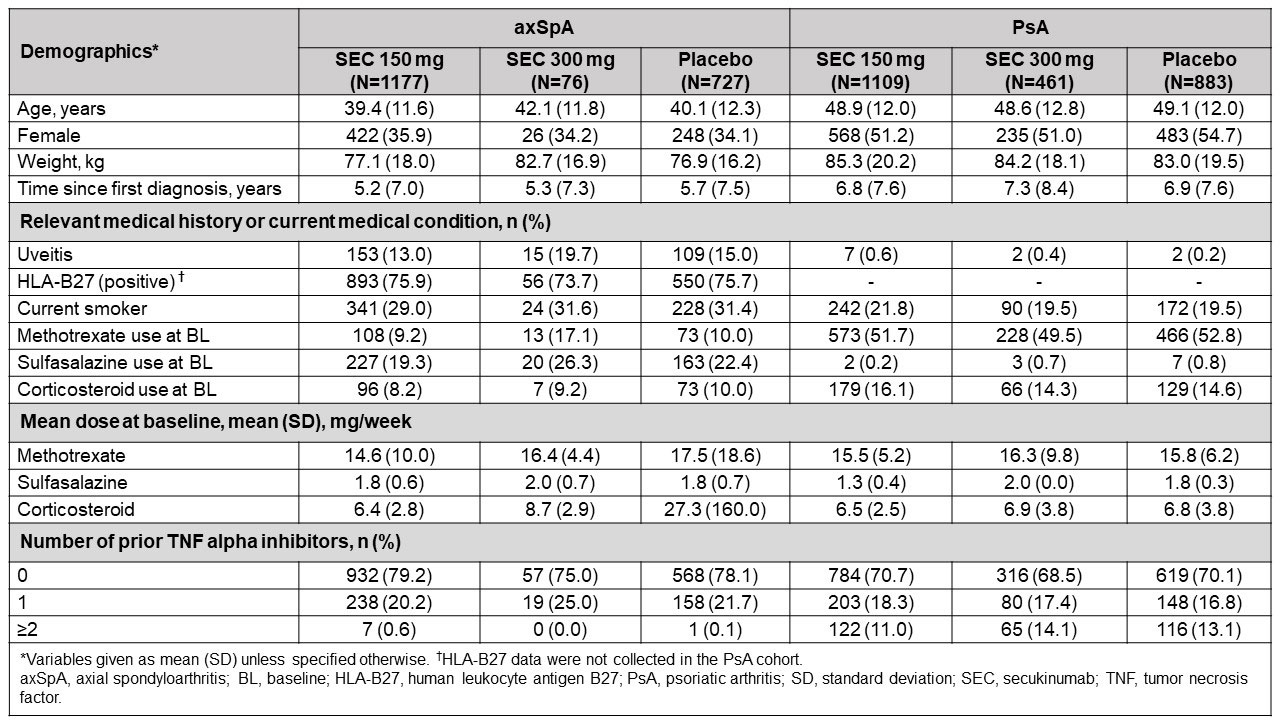
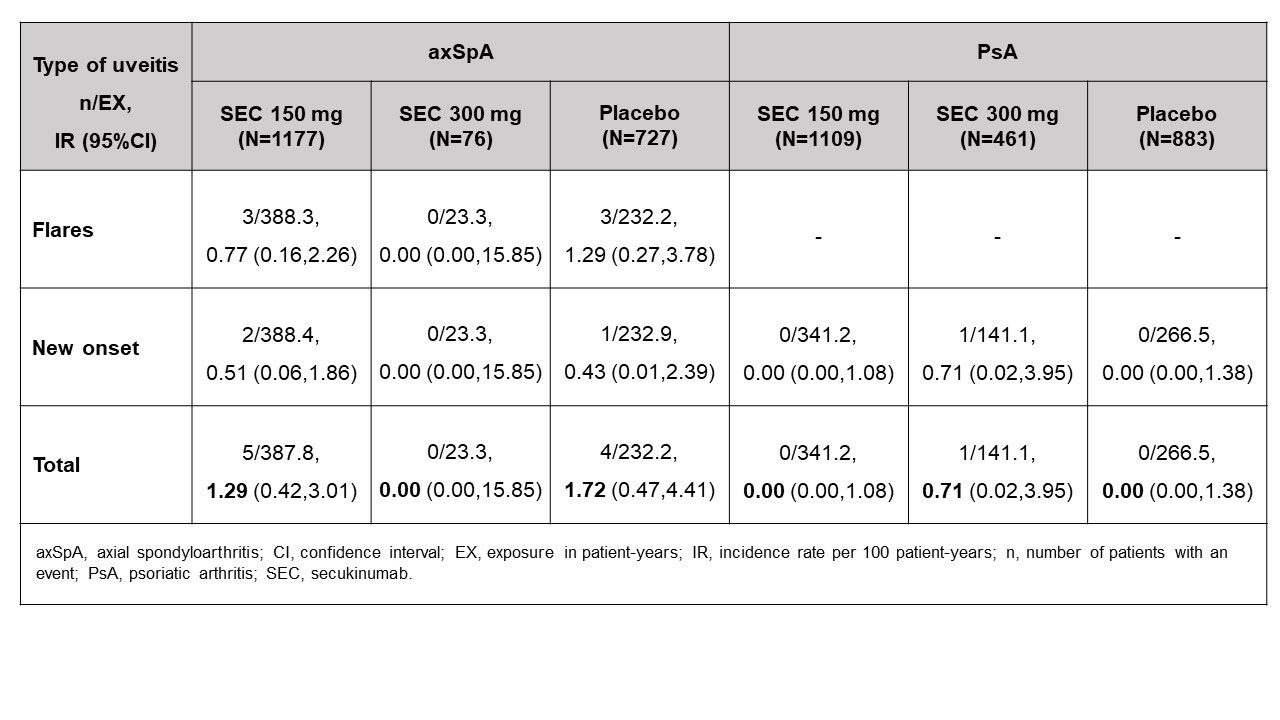
Results: Overall, 1980 and 2453 patients were included in the axSpA and PsA cohorts, respectively. Approximately 35% of the enrolled axSpA and 52% of the PsA patients were female. In the axSpA cohort, 14% of patients had history of uveitis, whereas a lower percentage (0.4%) had history of uveitis in the PsA cohort. In the axSpA and PsA cohorts, 79% and 70% of patients, respectively, were naïve to TNF alpha inhibitors. Twenty one percent of the axSpA patients received sulfasalazine, while 52% of the PsA patients received methotrexate, concomitantly (Table 1). In patients with axSpA, the incidence rate for uveitis was 1.29 per 100 patient-years in the secukinumab 150 mg arm (5 events [3 flares; 2 new-onset]) and 1.72 per 100 patient-years in the placebo arm (4 events [3 flares; 1 new-onset]). However, no cases were observed in the secukinumab 300 mg arm. Moreover, in the PsA cohort, the incidence rate for uveitis was 0.71 per 100 patient-years in the secukinumab 300 mg arm (1 new-onset event); however, no events were reported in the secukinumab 150 mg and placebo arms (Table 2).
Conclusion: In this comparative phase analysis, low incidence of uveitis (including new onset of cases and flares) was observed in patients with axSpA and PsA treated with secukinumab and placebo. These findings are consistent with previous reports1 and similar to real-world evidence data on uveitis in axSpA and PsA patients treated with secukinumab.5
References:
- Deodhar A, et al. ACR Open Rheumatol. 2020;2(5):294–9
- Sharma S, Jackson D. Best Pract Res Clin Rheumatol. 2017;31(6):846–62
- Roche D, et al. Arthritis Res Ther. 2021;23:192. https://doi.org/10.1186/s13075-021-02549-0
- Cantini F, et al. J Rheumatol Suppl. 2015;93:27–9
- Kiltz U, et al. Arthritis Rheumatol. 2021;73 (suppl 10)
To cite this abstract in AMA style:
Brandt-Juergens J, Rudwaleit M, Behrens F, Ritchlin C, Peterlik D, Quebe-Fehling E, Calheiros R, Deodhar A. Uveitis in Patients with Axial Spondyloarthritis or Psoriatic Arthritis: Baseline Characteristics and Incidence Rates During Secukinumab and Placebo Comparative Phase: A Post Hoc Analysis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/uveitis-in-patients-with-axial-spondyloarthritis-or-psoriatic-arthritis-baseline-characteristics-and-incidence-rates-during-secukinumab-and-placebo-comparative-phase-a-post-hoc-analysis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/uveitis-in-patients-with-axial-spondyloarthritis-or-psoriatic-arthritis-baseline-characteristics-and-incidence-rates-during-secukinumab-and-placebo-comparative-phase-a-post-hoc-analysis/