Session Information
Date: Saturday, November 12, 2022
Title: Vasculitis – Non-ANCA-Associated and Related Disorders Poster I: Giant Cell Arteritis
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
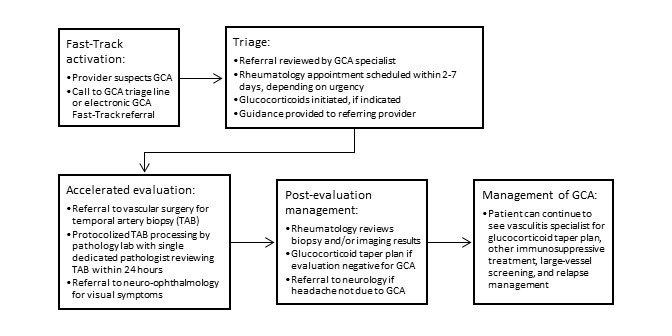
Background/Purpose: The Giant Cell Arteritis (GCA) Fast-Track program was implemented at our center with the goal of accelerating diagnosis of this rare and rapidly progressive disease. The main components of the program are outlined in Figure 1. Prior studies have demonstrated that GCA fast-track programs improve outcomes. It is unclear whether expedited, specialized care delivered through a fast-track process improves quality of care and outcomes in GCA independent of temporal artery ultrasound. This study evaluated our GCA fast-track program with the hypothesis that rapid, multi-disciplinary care of patients with suspected GCA enables achievement of goals outlined by clinical management guidelines without the use of temporal artery ultrasound.
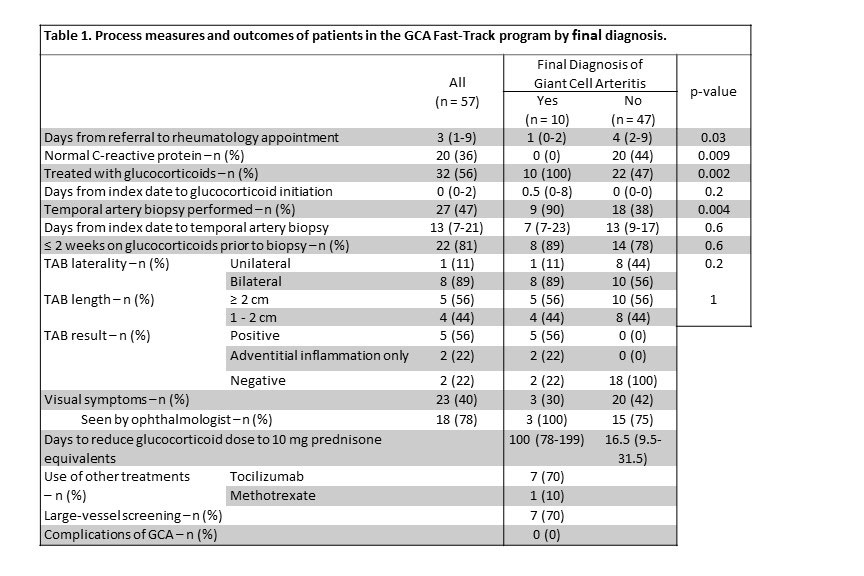
Methods: Patients referred to our GCA Fast-Track program between January 2020 and January 2022 were included. Patients were excluded from analysis if they had a previous diagnosis of GCA or incomplete medical records. Process measures included time to rheumatology evaluation, use of glucocorticoids, temporal artery biopsy, and large-vessel screening as per published guidelines. Complications of GCA were defined as permanent vision loss, large-vessel manifestations (aneurysm, stenosis, dissection), or death. Groups were compared using the Wilcox rank-sum test or Fisher’s exact test.
Results: Among 65 patients referred to the program, 57 patients were included in the analysis summarized in Table 1. Nine lower risk patients were determined not to need a rheumatology appointment after initial triaging. Patients who were ultimately diagnosed with GCA were seen more quickly by rheumatology after referral than those who were not diagnosed with GCA (1 day vs. 4 days, p=0.03). 47% of patients underwent temporal artery biopsy (TAB) with the overall TAB positivity rate being 26%. Most patients seen through this program met the standards outlined in the American College of Rheumatology guidelines. All patients diagnosed with GCA that experienced visual symptoms were seen by an ophthalmologist. None of the patients diagnosed with GCA through the fast-track experienced permanent vision loss or any other disease-related complications. Normal C-reactive protein was observed in 0% of GCA vs 44% of non-GCA groups during evaluation for GCA.
Conclusion: Our Fast-Track program provided high-quality, comprehensive care for patients undergoing evaluation for GCA without relying on temporal artery ultrasound. Effective triaging enabled appropriate use of resources and no patients experienced disease-related complications including permanent blindness during the follow-up period. Future use of normal pre-treatment C-reactive protein to refine the triaging process may also be considered. Although the COVID-19 pandemic impacted the volume of patients and operations at our center, these data are likely generalizable to a non-pandemic situation. Further studies are needed to compare outcomes between conventional and fast-track pathways.
To cite this abstract in AMA style:
Costeas C, Banerjee S, Tamhankar M, Amudala N, Merkel P, Rhee R. Utilization of a Giant Cell Arteritis Fast-Track Program Independent of Ultrasound at a Single Center [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/utilization-of-a-giant-cell-arteritis-fast-track-program-independent-of-ultrasound-at-a-single-center/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/utilization-of-a-giant-cell-arteritis-fast-track-program-independent-of-ultrasound-at-a-single-center/