Session Information
Date: Monday, October 22, 2018
Title: Systemic Lupus Erythematosus – Clinical Poster II: Biomarkers and Outcomes
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
Measurement of treatment response in SLE clinical trials has been based on measurement of change from baseline; however a treat-to-target analysis has seldom been applied. The Lupus Low Disease Activity State (LLDAS), a potential response indicator for lupus clinical trials, has been found to correlate with reduced damage accrual in SLE1, indicating it may be a useful treatment target in the clinic. In a trial setting, LLDAS correlated with key outcome measures and discriminated responders from non-responders in a post-hoc analysis of the phase IIb MUSE trial of anifrolumab2.
We evaluated the utility of LLDAS in this post-hoc analysis of the BLISS-523 and BLISS-764 trials of intravenous belimumab in patients with moderate-severe SLE.
Methods:
LLDAS attainment was assessed at baseline and week 52. LLDAS is defined as having all of the following: a) SLEDAI-2K≤4 without major organ activity; b) no new disease activity; c) physician global assessment of activity score (PGA, 0-3)£1; d) prednisolone dose ≤7.5mg/day; and e) standard immunosuppressants allowed. Attainment of LLDAS, association with the primary trial endpoint (SRI-4), discrimination between belimumab and placebo-treated patients, and predictors of LLDAS attainment, were evaluated using descriptive statistics and the Chi-square test, using R (v3.4.3).
Results:
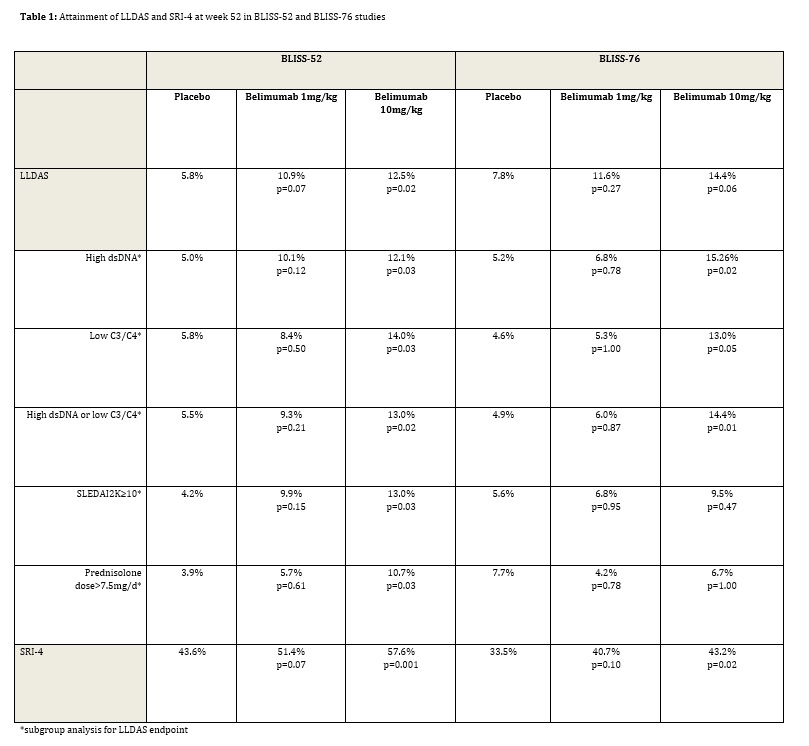
Few patients were in LLDAS at study entry (0-2.2%). At week 52, in both studies, fewer patients attained LLDAS compared to the SRI-4 (Table 1). At week 52, for belimumab 10mg/kg, 17.0% of patients in BLISS-52 and 19.3% of patients in BLISS-76 who achieved an SRI-4 also attained LLDAS. In BLISS-52, significantly more patients attained LLDAS at week 52 on belimumab 10mg/kg compared to placebo (12.5% vs 5.8%, p=0.02), with a near significant difference at the same time-point in BLISS-76 (14.4% belimumab 10mg/kg vs placebo 7.8%, p=0.06). Numerically more patients were in LLDAS at week 52 for both studies for the belimumab 1mg/kg group compared to placebo. In subgroup analysis, LLDAS attainment at week 52 was more likely on belimumab 10mg/kg than placebo in patients with high anti-dsDNA antibody levels ³30 IU/ml (both studies), low C3 (<90mg/dl)/C4 (<16mg/dl) (both studies), high anti-dsDNA antibody levels or low complement levels (both studies), SLEDAI-2K≥10 (BLISS-52), or prednisone dose ≥7.5mg/d (BLISS-52) at study entry.
Conclusion:
In BLISS-52, LLDAS was able to discriminate responders from non-responders in the belimumab 10mg/kg group. Fewer patients met LLDAS criteria at week 52 compared to the SRI-4, suggesting that LLDAS is a more stringent measure of treatment response than the SRI-4. Our findings support the discriminant validity of LLDAS as a useful outcome measure in SLE RCTs.
- Franklyn K, et al. Ann Rheum Dis. 2016;75:1615-21
- Morand E, et al. Ann Rheum Dis. 2018;77:706-13
- Navarra S, et al. 2011;377:721-31
- Furie R, et al. Arthritis Rheum. 2011;63:3918-30
To cite this abstract in AMA style:
Oon S, Huq M, Golder V, Ong E, Morand E, Nikpour M. Utility of the Lupus Low Disease Activity State (LLDAS) in Discriminating Responders in the BLISS-52 and BLISS-76 Phase 3 Trials of Intravenous Belimumab in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/utility-of-the-lupus-low-disease-activity-state-lldas-in-discriminating-responders-in-the-bliss-52-and-bliss-76-phase-3-trials-of-intravenous-belimumab-in-systemic-lupus-erythematosus/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/utility-of-the-lupus-low-disease-activity-state-lldas-in-discriminating-responders-in-the-bliss-52-and-bliss-76-phase-3-trials-of-intravenous-belimumab-in-systemic-lupus-erythematosus/