Session Information
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Guidelines for repeat Latent Tuberculosis Infection (LTBI) testing while on biologics are not clearly defined. The American College of Rheumatology recommends repeat LTBI screening only in persons with risk factors for tuberculosis(TB) whereas the National Psoriasis Foundation recommends repeat annual LTBI screening in all patients on biologics. Furthermore, recommendations for annual LTBI screening for patients on biologics have been incorporated into the Medicare Merit-Based Incentive Payment Systems and will impact physician reimbursement. However, little evidence supports that this practice of repeat annual screening is clinically valuable and/or cost-effective in patients on biologics.
Objective: To determine the value and cost-effectiveness of serial LTBI screening in patients taking biologics and to identify risk factors in patients who convert from negative to positive QuantiFERON TB test (QFT) results while on biologics.
Methods: We retrospectively reviewed LTBI screening results in patients treated with biologics for chronic inflammatory/autoimmune conditions at a single, tertiary care center between August 2007 and March 2019. For each patient, we collected demographic information, primary underlying diagnosis, biologics used, length of biologic therapy, number of QFTs and QFT results. Patients without repeat QFT results following biologic initiation and/or all QFTs outside the treatment period with biologics were eliminated from the study.
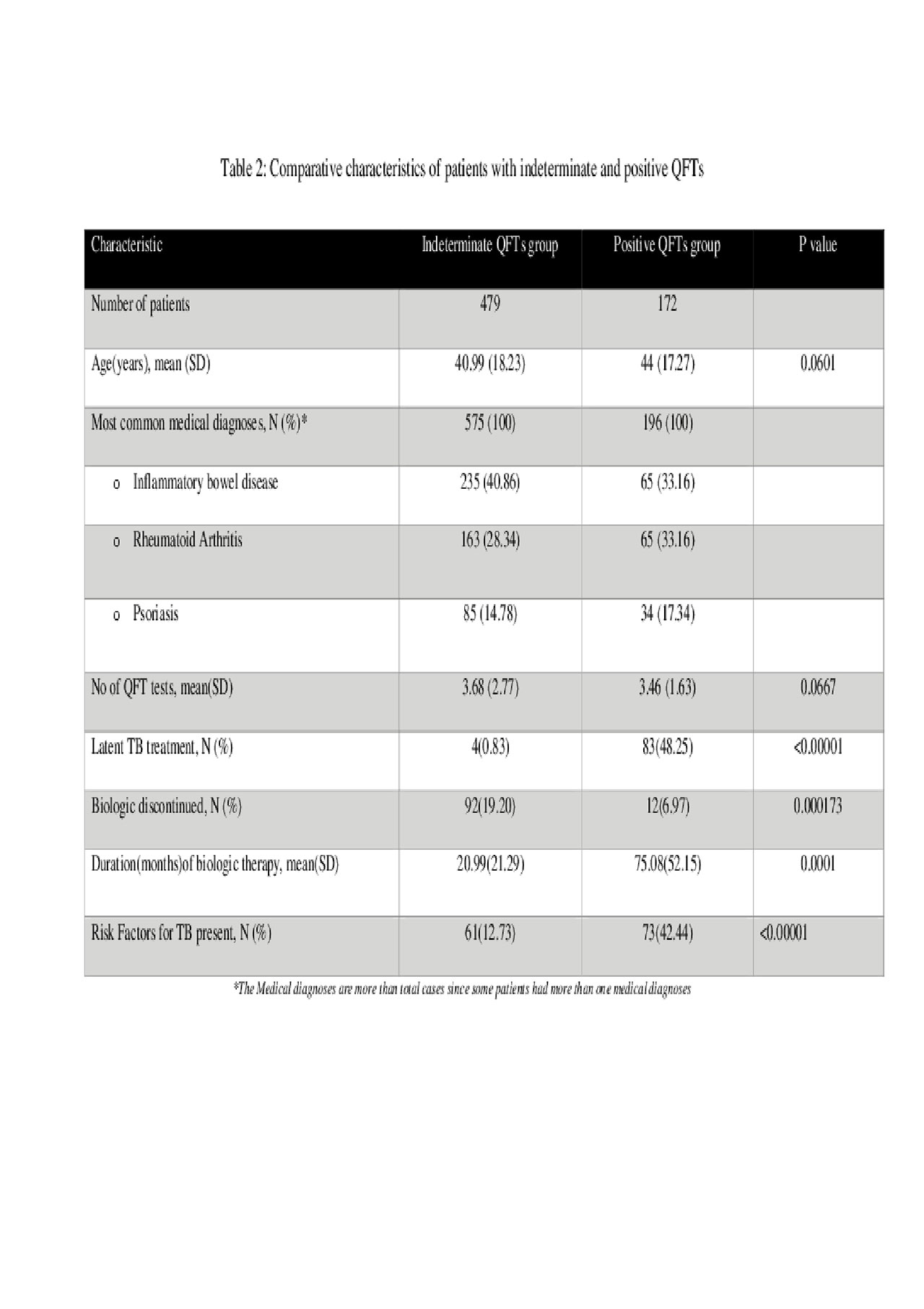
Results: Of 10,914 patients treated with biologics and with QFT results, 5212 had >1 repeat QFT result after starting biologic therapy (mean 3.2 per patient) and were included in our study (Table 1). The most common medical diagnosis in the study cohort were Inflammatory Bowel Disease (31%), Rheumatoid Arthritis (29%) and Psoriasis +/- Psoriatic Arthritis (25%). Majority patients had all negative QFTs (87.5%, n=4561), 9.2 % patients (n=479) had >1 indeterminate QFT and 3.3% patients (n=172) had >1 positive QFT (Table 2). Amongst patients with positive QFTs, only 61 patients converted from a negative to a positive QFT after initiation of biologic therapy. Importantly, only one case of active TB was found in the entire study cohort and this patient had a significant risk factor in the form of recent travel to a TB endemic area.
Conclusion: This represents the largest single institution study evaluating rates of QFT test positivity/conversion in patients taking biologics. Repeat LTBI testing in patients taking biologics revealed a low rate of conversion (1.17%), suggesting low clinical value and an undesirable cost-benefit ratio. Additionally, a high percentage of positive QFT converters had risk factors for TB exposure. Our results suggest clinical utility and cost-effectiveness of repeat LTBI screening in patients on biologics may be more valuable if not performed routinely, but driven by a focused review of TB exposure risk factors in each patient.
To cite this abstract in AMA style:
Khanna U, Ellis A, Galadari A, Hu J, Gallop J, Husni M, Fernandez A. Utility of Repeat Latent Tuberculosis Testing in Patients Taking Biologics [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/utility-of-repeat-latent-tuberculosis-testing-in-patients-taking-biologics/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/utility-of-repeat-latent-tuberculosis-testing-in-patients-taking-biologics/