Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Idiopathic inflammatory myopathies (IIM) are chronic autoimmune diseases affecting multiple organ systems and associated with a diverse autoantibody profile. Anti-SSA/SSB are the most frequent myositis associated antibodies. Historically, anti-Ro/SSA reporting has been combined for autoantigens Ro 52 and Ro 60 kDa. Recent data suggest that anti-Ro 52 and anti Ro 60 differ significantly in their clinical associations. Correlation of anti-Ro 52 with higher frequency of ILD has been demonstrated, but not been verified for commercially available anti-SSA/SSB assay.
Aim: To compare commercially available anti-SSA/SSB assay positivity and anti-Ro52 assay positivity as determinants of Interstitial Lung Disease (ILD) in IIM.
Methods: We queried the Northwell Myositis Center database for patients with IIM and available data for anti SSA/SSB and anti Ro52 between 1/1/2007 to 4/6/2018. All patients met 2017 EULAR/ ACR classification criteria for IIM. Anti-SSA and anti-SSB was measured by commercially available multiplexed bead-based immunoassay. Anti-SSA and anti-SSB were combined and analyzed as a single group because SSB was only found in association with positive SSA in our cohort. Anti-Ro52 was measured by commercially available immunoenzymatic assay (EIA). Patients were divided in 6 groups based on positivity/availability of each test (Table1). The frequency of ILD was calculated for each group. Statistical analyses included Chi-square, Fisher’s Exact test, and Wilcoxon Rank Sum test to determine statistical differences in group distributions and McNemar’s test was performed to compare groups
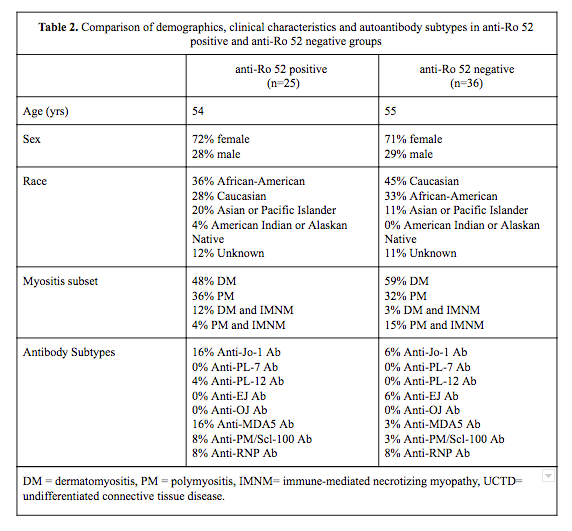
Results: There were 108 patients that met criteria for inclusion of which 31% (34/108) were anti-SSA/SSB positive. Anti-Ro 52 data were available for 61 patients and was positive in 41% (25/61). Some Ro 52 positive patients were nested in SSA/SSB negative group 36% (9/25) but majority of these had low titers of Ro 52. Both Ro 52 positive and negative patient groups had similar distribution for age, gender and race, as well as subtypes of IIM. Demographics, clinical characteristics and distribution of myositis associated antibodies in both groups are listed in Table 2.
As anticipated, the frequency of ILD was significantly higher in anti-Ro 52 positive compared with anti-Ro52 negative patients (64% vs 25%, p value= 0.0041). Similarly, anti-SSA/SSB positive status was associated with higher rate of ILD, independent of anti-Ro 52 status (56% vs 28%, p = 0.0084). Likewise, when patients with unknown anti-Ro 52 status were excluded from analysis, the difference remained statistically significant although weaker (55% vs 33%, p = 0.0038 ). There was no statistically significant difference in frequency of ILD between anti-Ro52 positive and anti SSA/SSB positive individuals (64% vs 56% p=0.5).
Conclusion: Both commercially available assays for anti-SSA/SSB and anti-Ro 52 positivity conferred the increased rate of ILD in our IIM cohort. Our data suggests that commercially available anti SSA/SSB assay can be used as a surrogate to the Ro52 assay to determine risk of ILD in IIM in clinical practice.
To cite this abstract in AMA style:
Marder G, Narain S, Barilla-Labarca M, Valle A. Utility of Anti-SSA/SSB Assay and Anti-Ro 52 Antibody Assay in Routine Clinical Practice for Risk Assessment of Patients with Idiopathic Inflammatory Myositis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/utility-of-anti-ssa-ssb-assay-and-anti-ro-52-antibody-assay-in-routine-clinical-practice-for-risk-assessment-of-patients-with-idiopathic-inflammatory-myositis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/utility-of-anti-ssa-ssb-assay-and-anti-ro-52-antibody-assay-in-routine-clinical-practice-for-risk-assessment-of-patients-with-idiopathic-inflammatory-myositis/