Session Information
Date: Monday, November 11, 2019
Title: 4M119: Sjögrenʼs Syndrome – Basic & Clinical Science (1902–1907)
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: To use ultrasound (US) to demonstrate that ianalumab, a monoclonal antibody with dual mechanisms-of-action of BAFF:BAFF-R blockade and enhanced, ADCC-mediated B cell depletion, can modulate the inflammation and morphology within salivary glands of patients with primary Sjögren’s syndrome (pSS).
Methods: In a single-center study, 27 patients fulfilling the revised American European consensus criteria for pSS with stimulated whole salivary flow rate of >0 mL/minute and active disease (EULAR Sjögren’s Syndrome Disease Activity Index; ESSDAI ≥6) were randomly assigned between three treatment groups in a 24-week double-blind treatment period to receive a single i.v. dose of either 3 mg/kg or 10 mg/kg ianalumab, or placebo1. Concurrent with clinical and laboratory outcomes that included ESSDAI, Physician’s Global Assessment, and patient-reported outcomes for disease activity, fatigue and quality of life ( EULAR Sjögren’s Syndrome Patient Response Index, Multi-Dimensional Fatigue Index, Short Form-36, Patient’s Global Assessment), multi-modal US images were acquired at baseline and weeks 6, 12, and 24. Using a single US device, applied modalities included: 1) echostructure by B-mode scored 0-4 in 5 categories according to de Vita classification2,3, 2) large vessel blood flow by power Doppler (vascularity index, scored 0 for no vascularization and 1-3 for mild, moderate and high vascularization, respectively), and, limited to parotid glands (PG) only, 3) microvascularization using contrast-enhanced US (area under the curve, time to peak) and 4) gland stiffness by sonoelastography4,5.
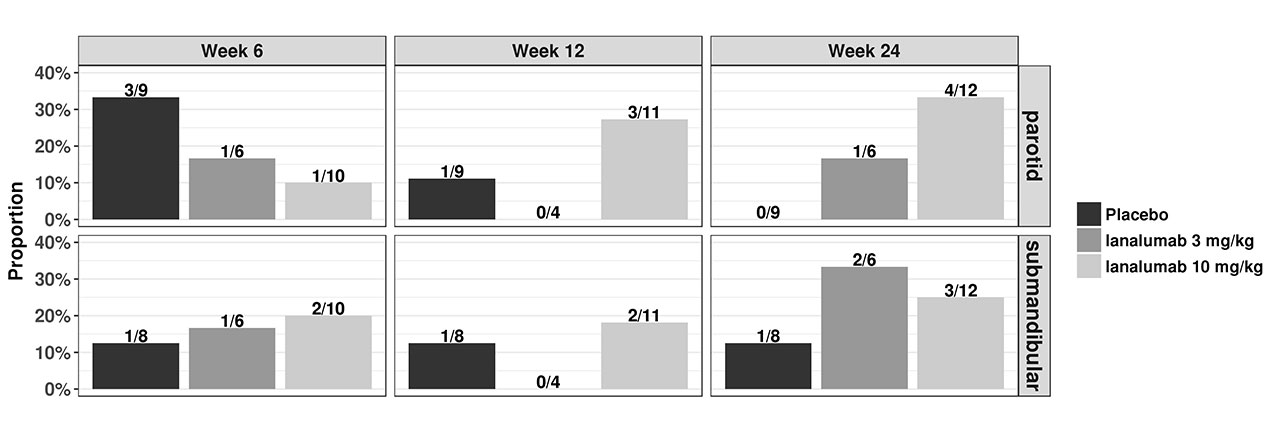
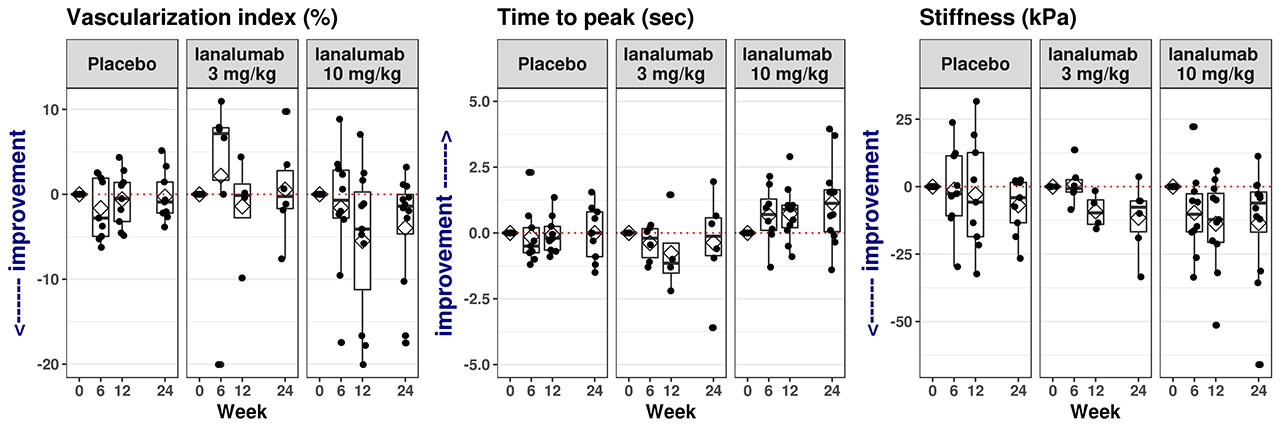
Results: The overall profiles of US data differed greatly between PG and submandibular glands (SMG) but were comparable between the respective left and right sides of these glands. A numerical improvement in both PG and SMG quality and declining inflammation in the glands were observed, including more de Vita responders (patients achieving ≥1 point reduction from baseline) in the VAY736 groups than in placebo (Figure 1), and changes in perfusion of both PG and SMG, as shown from large vessels and microvascular assessments (Figure 2), and a trend of decrease in PG stiffness (Figure 2). Change in some US parameters is observed to have correlation with some clinical endpoints, including change in thickness with change in ESSDAI at week 12 (Spearman correlation coefficient = -0.63, p value = 0.0031), and change in TTP at week 12 correlated with change in patient global assessment (Spearmann cc=0.61; p=0.018) and with MFI-physical fatigue (Spearman cc = 0.61, p-value = 0.046).
Conclusion: Early signs of salivary gland improvement in response to an effective intervention can be shown using non-invasive, comprehensive, ultrasound-based approach at multiple time points without the need of biopsies.
To cite this abstract in AMA style:
Diekhoff T, Posch M, Wagner F, Fischer T, Schefer Q, Dörner T, Laurent D, Li Y, Oliver S. Using Multi-modal Ultrasound to Assess Disease Activity Within the Salivary Glands of Patients with Primary Sjögren’s Syndrome Treated with Ianalumab (VAY736) [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/using-multi-modal-ultrasound-to-assess-disease-activity-within-the-salivary-glands-of-patients-with-primary-sjogrens-syndrome-treated-with-ianalumab-vay736/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/using-multi-modal-ultrasound-to-assess-disease-activity-within-the-salivary-glands-of-patients-with-primary-sjogrens-syndrome-treated-with-ianalumab-vay736/