Session Information
Date: Tuesday, November 9, 2021
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster II (1862–1888)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Disease activity assessment can be challenging in Takayasu’s arteritis (TAK), which can lead to difficulty in determining eligibility for enrollment into randomized clinical trials (RCTs). FDG-PET can complement clinical assessment of disease activity. The study objective was to determine whether incorporation of PET data, in addition to clinical assessment, influences decisions about trial enrollment in TAK.
Methods: An online survey was completed by physicians with expertise in TAK. Participants were asked background questions, including personal experience with use of PET. Clinical vignettes of commonly-encountered clinical scenarios were presented, based on actual cases derived from a prospective, observational cohort. In part A of each vignette, clinical symptoms, examination findings, acute phase reactant (APR) levels, and angiographic data were presented. Participants were asked if there was sufficient evidence of active vasculitis to enroll into a RCT studying a new treatment agent and to rate level of confidence about their assessment (scale of 0-100). In part B, detailed information from the same patient’s PET scan was presented and participants were again asked whether they would enroll the patient into a RCT and level of confidence about their assessment. Generalized linear mixed models, adjusting for correlated data, were used to evaluate associations between PET activity, APR levels, and enrollment decisions.
Results: Sixty-eight vasculitis experts completed the survey. The majority of physicians were from Europe [n=45 (66%)] or North America [n=16 (24%)] and most were rheumatologists [n=54 (79%)]. Physicians had varying experience with managing patients with TAK: < 10 patients [n=15 (22%)], 10-40 patients [n=32 (47%)], and >40 patients [n=21 (31%)]. Most physicians reported using PET to diagnose TAK [n=56 (82%)] and to monitor disease activity [n=45 (66%)].
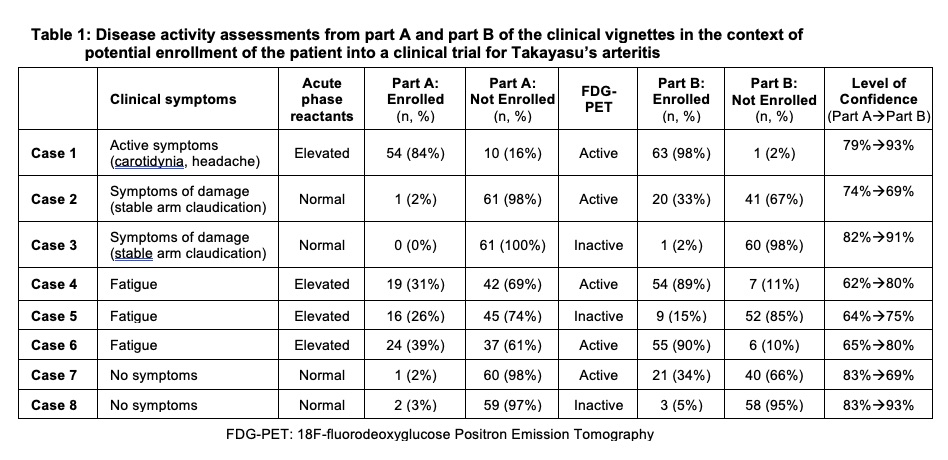
In part A of the vignettes, greater variability in trial enrollment decision was observed in cases of constitutional symptoms alone (e.g. fatigue) and elevated APR levels (Cases 4-6) (TABLE 1).
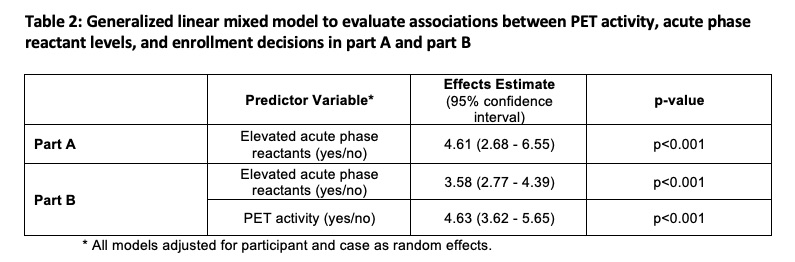
In Part B, level of confidence improved when PET findings aligned with clinical assessment from Part A (Case 1,3,8). In cases where PET findings did not align with clinical assessment in part A (e.g. absent clinical symptoms with active PET scan, “subclinical inflammation”), the degree of variability about whether to enroll/not enroll increased in Part B and level of confidence worsened (Case 2, 7). In cases with the highest variability about enrollment in part A (Cases 4-6), PET activity drove the decision of whether to enroll in Part B and level of confidence improved. PET activity and APR levels independently contributed to trial enrollment decisions (TABLE 2).
Conclusion: Physicians vary about which patients with TAK should be enrolled into RCTs. Incorporation of PET findings with clinical assessment influences trial enrollment decisions and improves physician confidence about disease activity assessment. Cases of subclinical inflammation pose unique challenges for trial enrollment. Future RCTs in TAK should consider incorporating PET findings into eligibility criteria.
To cite this abstract in AMA style:
Quinn K, Alessi H, Rose E, Ahlman M, Redmond C, Luo Y, bolek E, Langford C, Ponte C, Merkel P, Grayson P. Using 18F-fluorodeoxyglucose Positron Emission Tomography to Standardize Clinical Trial Recruitment in Takayasu’s Arteritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/using-18f-fluorodeoxyglucose-positron-emission-tomography-to-standardize-clinical-trial-recruitment-in-takayasus-arteritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/using-18f-fluorodeoxyglucose-positron-emission-tomography-to-standardize-clinical-trial-recruitment-in-takayasus-arteritis/