Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Rituximab (RTX) is an effective therapy for ANCA-Associated Vasculitis (AAV). The Canadian Vasculitis Research Network (CanVasc) recommends RTX for induction among patients with unacceptable risk of infertility or other contraindication to cyclophosphamide, and among those who have not responded or have relapsed after conventional induction therapy. RTX is also recommended for maintenance, where available. Our objectives were to (1) describe real-world use of RTX in AAV since the publication of the Rituximab in ANCA-associated vasculitis (RAVE) trial in 2010, and (2) compare current RTX use for AAV across regions, sexes, and age groups.
Methods: Anonymized, aggregate-level data on RTX use in AAV (Granulomatosis with Polyangiitis and Microscopic Polyangiitis) from January 2010-December 2018 were obtained from the national pharmaceutical-funded patient support program database, covering approximately 85% of RTX prescriptions for vasculitis in Canada and excluding inpatient infusions. RTX use was compared across sexes, age groups, geographic regions, and coverage type (private, public, or combination). The number of unique applications and denials (all funding sources), and new drug starts were assessed over time. Data on RTX indication (induction, relapse, or maintenance) were not available.
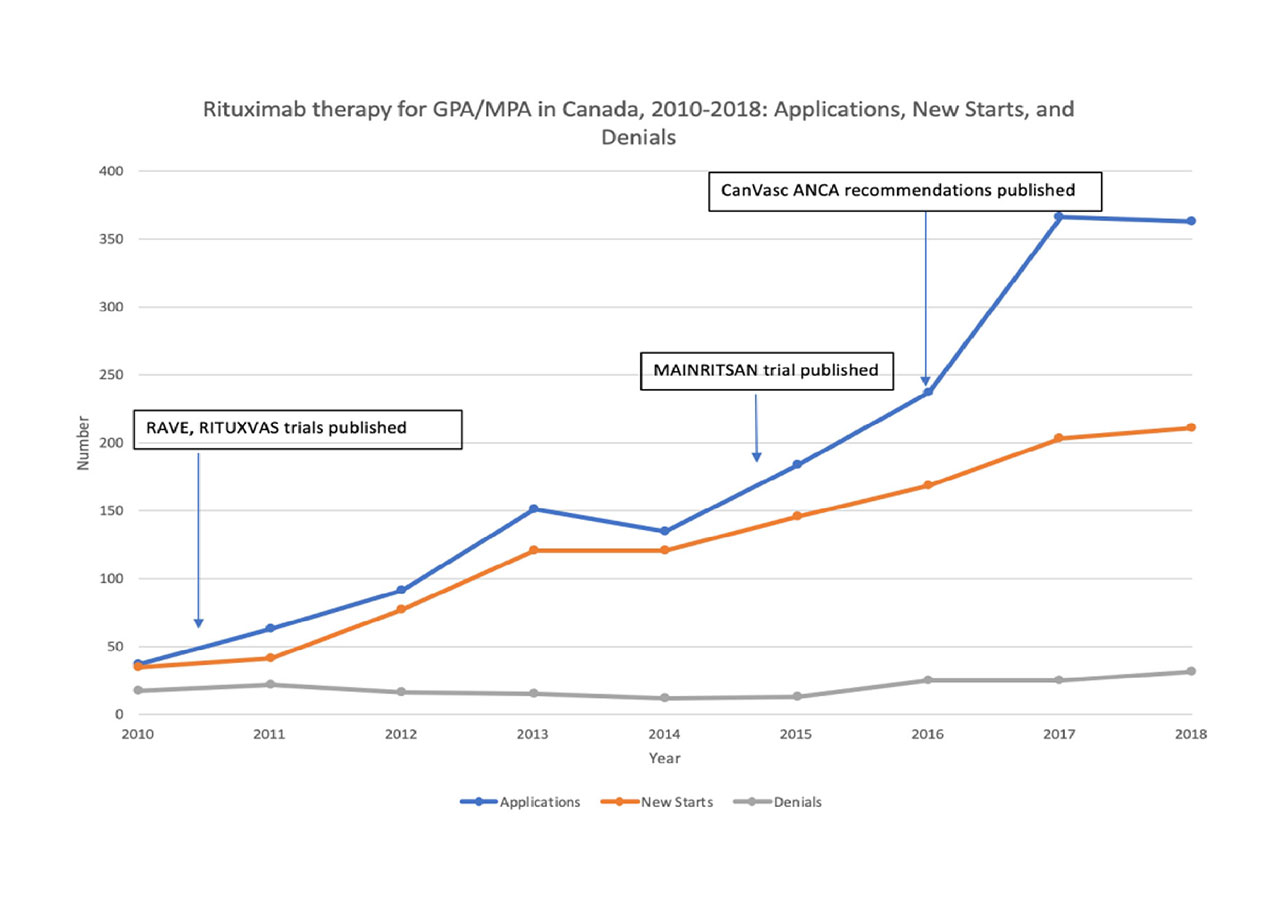
Results: From 2010, 1124 patients started RTX therapy for AAV (including new diagnosis, relapsing disease, or maintenance). New RTX starts increased 6-fold, from 35 in 2010 to 211 in 2018, while the number of application denials (from public and/or private funders) remained relatively stable (from 18 in 2010 to 32 in 2018). Applications for treatment of AAV represented only 11% of the total RTX applications for other disease indications. Among current RTX users (n=556), 306 (55%) patients were female and 115 (21%) were of reproductive age (18-39 years). Patients received public coverage more often in Quebec (51% [95% CI, 42-60]) and Western provinces (46% [38-53]) compared to Ontario/Atlantic provinces (26%[22-32]).
Conclusion: The use of RTX for AAV in Canada has increased substantially since clinical trials established its efficacy. Between provinces, there are significant differences in the proportion of patients covered publicly, although this could reflect practice variations in the use of RTX for maintenance (not currently covered publicly). Only 21 % of RTX users were in the reproductive age group, suggesting that RTX is used broadly for AAV treatment and is not being limited to those with fertility concerns.
To cite this abstract in AMA style:
Mendel A, Carette S, Pagnoux C. Use of Rituximab for the Treatment of ANCA-Associated Vasculitis in Canada, 2010-2018 [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/use-of-rituximab-for-the-treatment-of-anca-associated-vasculitis-in-canada-2010-2018/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-rituximab-for-the-treatment-of-anca-associated-vasculitis-in-canada-2010-2018/