Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
ANCA-associated vasculitis (AAV) is a group of rare systemic vasculitidies with significant morbidity and mortality.
Plasma exchange (PLEX) has been shown to decrease risk for end-stage renal
disease in adults with severe AAV, however there are no data in children. We aimed to characterize the use of
PLEX in children with AAV hospitalized in the United States.
Methods: We conducted a retrospective cohort
study of children hospitalized with AAV from 2004-2014 utilizing the Pediatric
Health Information System database which contains inpatient administrative and
billing data from 47 hospitals in the United States. The cohort included
patients with the ICD-9-CM code 446.4 as one of the discharge diagnoses and ≥1
charge for glucocorticoids. Cyclophosphamide and rituximab exposure was
determined using pharmacy billing data. Receipt of PLEX was determined using
ICD-9-CM and clinical transaction classification codes. Demographics and
clinical variables were summarized using count & percentage or median &
interquartile range as appropriate. We built a multivariate mixed effects regression
model accounting for clustering by hospital using a backwards selection model starting
with preset variables (age, sex, rituximab, cyclophosphamide, mechanical
ventilation, dialysis, year, & hospital case load of AAV) to determine
factors associated with use of PLEX.
Results: During the study period there were 1290
admissions for 393 children with AAV at 43 hospitals. Median age at first
admission was 14.6 (IQR 12.2,16.1); 60.6% were female.
236 (60%) children required readmission with a median of 2 readmissions (IQR 1,
3). The median length of stay for first admission was 9 days (IQR 4,15). The first readmission was a median of 55 days (IQR 12,
165) after initial discharge, with a median duration of 3 (IQR 2,6) days. During the first admission, 60 (15.5%) children
needed dialysis, and 67 (17.1%) required mechanical ventilation. 222 (56.8%)
children received cyclophosphamide, 83 (21.2%) received rituximab, 40 (10.2%)
received both. Eighty-four children (21.5%) received PLEX, of whom 80 (95%)
received concomitant cyclophosphamide and/or rituximab. The
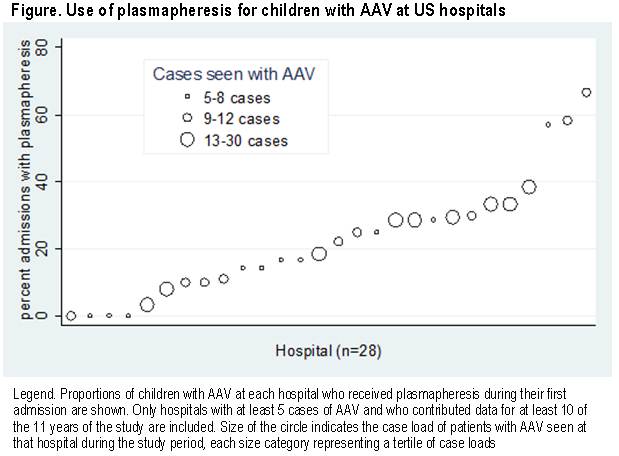
median proportion of children who received PLEX at each hospital with ≥5
cases of AAV was 0.20 (range 0-0.67) (Figure).
There was a significant increase in the use of PLEX for new patients over the
years of study (p=0.04). In multivariate regression, male sex, cyclophosphamide,
rituximab, and dialysis were associated with increased odds of receipt of PLEX.
Conclusion: PLEX use for treatment of AAV in
children is increasing over time, however its use
varies significantly between hospitals. Use of rituximab, cyclophosphamide, and
dialysis are associated with receipt of PLEX, confirming its use is reserved
for children with more severe manifestations. Future studies are needed to
assess the impact of PLEX on outcomes for children with AAV.
To cite this abstract in AMA style:
James K, Xiao R, Merkel PA, Weiss PF. Use of Plasma Exchange for Children Hospitalized with ANCA-Associated Vasculitis in the United States [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/use-of-plasma-exchange-for-children-hospitalized-with-anca-associated-vasculitis-in-the-united-states/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-plasma-exchange-for-children-hospitalized-with-anca-associated-vasculitis-in-the-united-states/