Session Information
Date: Monday, October 27, 2025
Title: (1612–1632) Vasculitis – Non-ANCA-Associated & Related Disorders Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Glucocorticoids(GCs) are the mainstay of treatment in Takayasu arteritis (TAK). Conventional DMARDs(cDMARDs) and biologic DMARDS(bDMARDs) are often used as steroid sparing agents in TAK. Janus kinase inhibitors (JAKi), recently shown to be effective in giant-cell arteritis may be effective to treat TAK,but evidence to date has been limited to small, single-center studies.In this worldwide multi-center study, we aimed to assess the efficacy and safety of JAKi in patients with TAK.
Methods: Patients fullfilling the 1990 ACR Classification Criteria for TAK were eligible to participate in a retrospective observational cohort study. Each patient must have been treated with a JAKi at any point in the disease course. A standardized case report form was completed by investigators at each participating tertiary referral center. Physician global assessment (PGA) was used as the gold standard definition of active disease, and the Kerr et al(NIH definition) was also used, if available. Complete remission was defined as no new signs and symptoms of vascular disease assessed by a physician, normalized acute phase reactants (APR) and reduction of GC dose under 10 mg/d of prednisolone or its equivalent at the third month of treatment. Partial response was defined as at least 50% decrease in PGA score,APR and at least 50% reduction of initial GC dose at the third month.
Results: Data was collected from 25 patients with TAK (mean age:34.5±11 years; female/male: 24/1;mean disease duration: 8.1±6 years) from 8 centers. All patients were refractory to both cDMARDs and bDMARDs, except 2 patients who were refractory only to cDMARDs and given JAKi prior to a trial of bDMARDs. Before switching to JAKi (19 tofacitinib, 6 upadacitinib), patients had received a median of 2 cDMARDs (range 1-4). Additionally, 23 (92%) patients received TNF inhibitors, 12(48%) tocilizumab, 3 (12%) secukinumab, 2 (8%) ustekinumab and 1 (4%) rituximab. The median duration of treatment with JAKi was 15.5(2-87) months. Remission was achieved in 13 (52%) patients in a median of 4(1-16) months, according to PGA.. At the 3rd month of the JAKi treatment, 6 (35%) of the patients were in complete remission while a partial response was observed in 2 (12%) patients. A significant decrease in the GC dose was also observed (GC baseline vs 3rd month:15 (0-60) vs 8 (0-30), p=0.002) At the end of the follow-up, GC dose was ≤ 4 mg in 16% (3/19) of patients. GC dose was increased in 1 patient (from 8 mg/d to 15 mg/d). GC was stopped in 3 (19%) patients. During follow-up 22%(4/18) of the patients experienced a relapse. Nine(36%) of the patients had a sustained remission.JAKi was discontinued in 11/23 (48%) patients.The main reason for discontinuation was disease activity in 7, infections in 2,unknown in 2 patients.Radiographic progression was observed in 4/21 (19%) patients during the follow-up.(Figure 1).
Conclusion: This study showed that JAKi achieved clinical remission in about half of patients with TAK refractory to DMARDs within a median 4 months of treatment. Complete remission was also achieved in 35% of the patients with a significant decrease in glucocorticoid dose in the 3rd month.Our results suggest that JAKi may be effective options for the treatment of patients with refractory TAK.
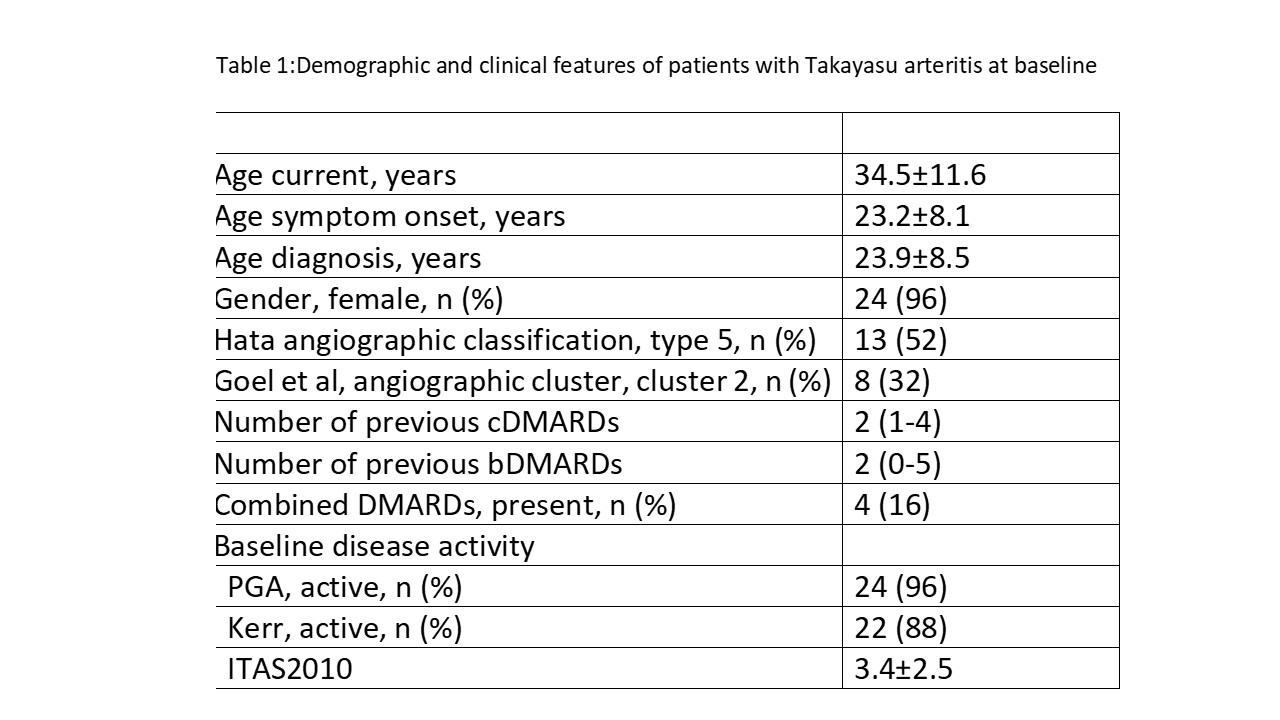 Table 1:Demographic and clinical features of patients with Takayasu arteritis at baseline
Table 1:Demographic and clinical features of patients with Takayasu arteritis at baseline
.jpg) Table 2. Follow- up data under JAK inhibitors
Table 2. Follow- up data under JAK inhibitors
.jpg) Figure 1. Drug survival of JAK inhibitors in patients with Takayasu arteritis.
Figure 1. Drug survival of JAK inhibitors in patients with Takayasu arteritis.
To cite this abstract in AMA style:
Alibaz-Oner F, Çakır İ, Tomelleri A, Avcu A, Quinn K, Misra D, Koster M, Grayson P, Hocevar A, Aksu K, Kerim D, Bolek E, Baldissera E, Campochiaro C, Dagna L, Kaymaz-Tahra S, Direskeneli H. Use of JAK inhibitors in patients with refractory Takayasu arteritis: A worldwide retrospective study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/use-of-jak-inhibitors-in-patients-with-refractory-takayasu-arteritis-a-worldwide-retrospective-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-jak-inhibitors-in-patients-with-refractory-takayasu-arteritis-a-worldwide-retrospective-study/
