Session Information
Session Type: Abstract Submissions (ACR)
TITLE: “Use of drug combinations in patients with osteoarthritis: a population-based cohort study.”
Background/Purpose:
Patients affected with osteoarthritis (OA) use different drugs in search for relief. We used a large database including medical records and pharmacy invoice data to explore use of drugs in OA patients in the period 2006-2010: non-steroidal anti-inflammatory drugs (NSAIDs), Symptomatic Slow Acting Drugs for OA (SYSADOA), COX-2 inhibitors (COX2i), opioids, and analgesics (paracetamol and metamizol).
Methods:
We screened the SIDIAP Database (www.sidiap.org) to identify those aged 40 years or older with a new diagnosis of OA, and ascertained use of NSAIDs, COX2i, SYSADOA, opioids, and analgesics in the period 2006-2010. SIDIAP contains anonymised medical records and pharmacy invoice data of a representative >4.9 million people in Catalonia (North-East Spain). We estimated prevalence (and 99% Confidence Intervals) of use of these drugs and their combinations, and the incidence (and 99%CI) of new users among newly diagnosed OA patients in the study period assuming a Poisson distribution.
Results:
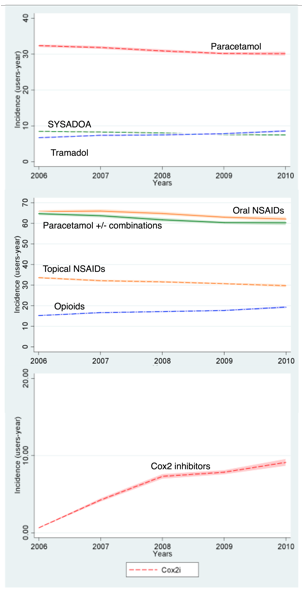
We identified 238,536 patients with an incident clinical diagnosis of OA. Among these, 128,655(53.9%) were treated with 3 or more drugs, and 60,740(25.5%) with 2 drugs. The most common combinations among the latter were:1.oral NSAID+analgesic, 2.topical NSAID+analgesic, and 3.oral NSAID+SYSADOA. Besides, 34,802(14.6%) patients received only 1 drug (the 3 most common being, in descending order: oral NSAID, analgesic and SYSADOA), and 14,339(6.0%) were not on pharmacologic treatment. Incidence of use of oral and topical NSAIDs, SYSADOA and analgesics decreased slowly from 2006 to 2010, while opioids increased continuously in the same period [Figure]. Incidence of use of COX2i rose steeply in the period 2006-2008, and continued increasing slowly from then onwards [Figure].
Conclusion:
About 75% of OA patients are treated with at least 2 drugs, and more than half receive 3 or more. Incidence of use of commonly used drugs (such as analgesics and topical NSAIDs) is decreasing, whilst prescriptions of opioids increase. In addition, use of COX2i continues to grow, yet more slowly after 2008, when warnings on the detrimental effects of COX2i on cardiovascular health were published. These data suggest that patients with OA are often polymedicated, and that current guidelines are poorly implemented in general practice. This has potential implications not only in terms of health care costs but also for patient safety.
Figure. Incidence rates (and 99%CI) of newly indicated drugs for OA in the period 2006-2010: a) The most commonly used analgesic (Paracetamol), SYSADOAs and the most commonly prescribed opioid (tramadol) [top]; b) Topical and oral NSAIDs, Paracetamol (alone and combined with other drugs), and Opioids; c) Cox 2 inhibitors
Disclosure:
D. Prieto-Alhambra,
None;
R. Morros,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-drug-combinations-in-patients-with-osteoarthritis-a-population-based-cohort-study/