Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Biologics have been used off-label in treatment of refractory polymyositis (PM) and dermatomyositis (DM). In this study we aimed to describe the use of biologics in patients with PM and DM based on national registries in Sweden.
Methods: Patients were identified by linking the national patient care registers, listing all in-patients and out-patient visits seen by specialists to the prescribed drug register, the Swedish Rheumatology Quality register (SRQ) and the Swedish Myositis Network (SWEMYONET). To retrieve information on demographics, effectiveness and safety we used information in SRQ, SWEMYONET and patient records. Effectiveness was based on an overall assessment of clinical outcome in the patient records, serum levels of creatine phosphokinase (CPK) and prednisolone dose. Clinical data were collected at start of treatment, at last follow up within 12 months or at discontinuation of biological treatment. Therapies stopped within 3 months were excluded from effectiveness evaluation. Safety was evaluated by the number of patients stopping treatment due to adverse events within 12 months.
Results: 63 patients treated with at least one biologic (36 PM, 27 DM, mean age 60 (12), 45 women, 18 men) were identified at 11 different centers between 2001 and 2011. All identified cases were refractory to previous medication with mean 2.5 (1.3) DMARDS beings used prior to start of biologic therapy. Multiple biological treatments were common with 7 patients treated with 2 different biologics and 7 patients treated with 3 different biologics.
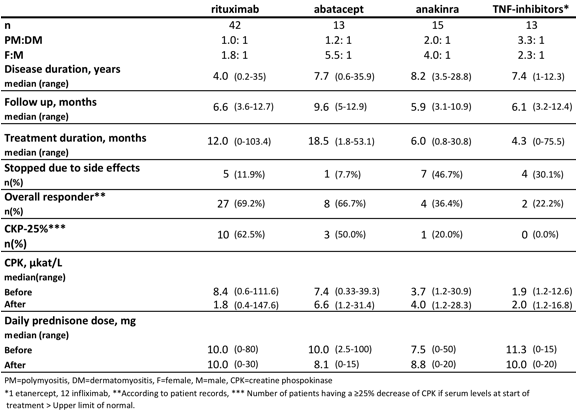
Rituximab (RTX) was the most often used biological agent (Table 1). No new treatment with anakinra (ANA) had been started after 2009 and no new treatment with TNF-inhibitors (TNFi) had been started after 2003. One treatment with tocilizumab was identified in 2011 but was stopped after 2 months due to a lung infection.
Table SEQ Table * ARABIC 1. Demographics and outcome measures of biologics in PM and DM
No statistical difference could be found for any of the biologics when comparing CPK level and prednisolone dose before and after treatment. 3 patients stopped their treatment due to disease remission. They were all treated with RTX. The most common adverse events leading to discontinuation were infections, and for ANA local injection reaction.
Conclusion: We found an increasing use of RTX and ABA in patients with PM and DM. In this population we found an overall favorable response to RTX and ABA in 65% of patients but less often to ANA and TNFi. A reduction of elevated CPK levels was observed more often after RTX and ABA treatment compared to ANA and TNFi treatment. RTX and ABA seemed to be well tolerated by patients.
Disclosure:
J. Svensson,
None;
A. Tjärnlund,
None;
B. Hanna,
None;
S. Magnusson Bucher,
None;
J. Askling,
None;
I. E. Lundberg,
BMS,
2,
Novartis Pharmaceutical Corporation,
5;
M. Dastmalchi,
None.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-biologics-in-polymyositis-and-dermatomyositis-a-national-register-study/