Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Use of biologics in arthritis patients with Hepatitis B and C : a multicentral retrospective case series
Abstract
Background/Purpose: Reactivation of viral hepatitis B (HBV) and C (HCV) has been reported in various case reports in arthritis patients on biological therapy. The objective of the study is to describe clinical characteristics and outcomes of arthritis patients with HBV or HCV treated with biological therapy.
Methods: This is a retrospective case series including 4 centers.
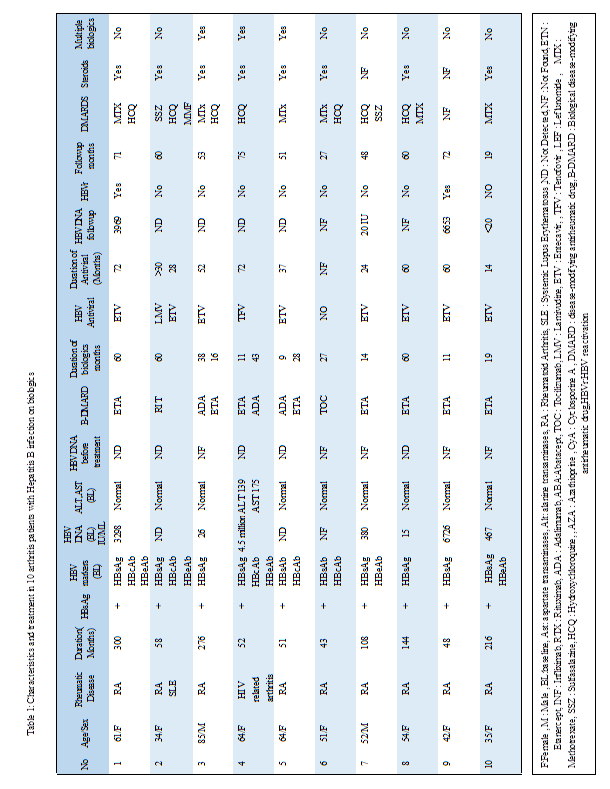
Results: Total of 20 arthritis patients with HBV and HCV on biological therapy were identified: 10 cases had (HBV) and 10 cases had (HCV) infection. In the HBV cohort the mean age was 51 (34-85) years, 80% were females. Most patients had Rheumatoid Arthritis (RA) 8(80%), 1 patient had RA/ Systemic lupus erythematosis (SLE) and 1 had HIV related arthritis.A total of 70% were inactive HBsAg carrier and the 3 had chronic hepatitis B. Prophylactic antiviral therapy was given in 9 patients with HBV cases: Entecavir (ETV) 7 , lamivudine and (ETV) 1 and tenofovir 1. The biologics used were Adalimumab (ADA), tociluzumab (TOC), rituximab (RIT) and etarnercept (ETA). There were 2 cases with chronic HBV had reactivation with no elevation of the transaminases.
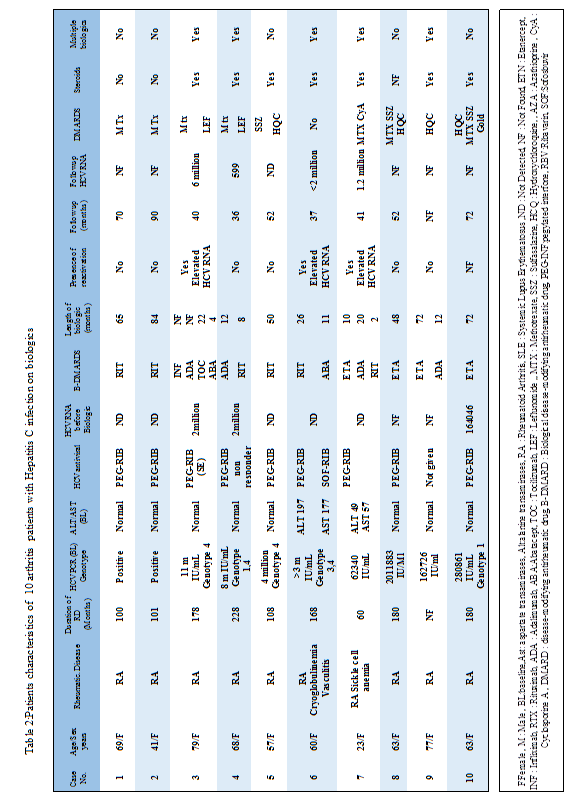
The mean age in Hepatitis C (HCV) cohort was 54 (23-79) years, all were female RA patients. 5 had detectable HCV RNA before start of biologics. Antiviral treatment was given in 9 (90 %) patients of the hepatitis C cohort: pegylated interferone plus ribavarin (PEG-RIB) 8, PEG-RIB and sofosbuvir plus ribavirin (SOF-RIB) 1pat. The biologics that were used: (ETA, ADA, TOC, RIT, abatacept (ABA) and infliximab (INF). There were 3 patients found to have elevated HCV RNA with elevation of transaminases during followup on biologics meeting criteria for hepatitis C reactivation. All three cases were genotype 4. One of the patients with HCV reactivation was started on SOF-RIB that showed undectable HCV RNA on follow-up.
Conclusion: We report a successful and safe use of biological therapy in patients with arthritis infected with HBV or HCV. Frequent monitoring is essential to detect reactivation that might occur.
To cite this abstract in AMA style:
Abdulaziz S, Halabi H, Omair M, Attar S, Shabrawishi M, Neyazi A, Alnazzawi H, Meraiani N, Almoallim H. Use of Biologics in Arthritis Patients with Hepatitis B and C : A Multicentral Retrospective Case Series [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/use-of-biologics-in-arthritis-patients-with-hepatitis-b-and-c-%e2%80%8f-a-multicentral-retrospective-case-series/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-biologics-in-arthritis-patients-with-hepatitis-b-and-c-%e2%80%8f-a-multicentral-retrospective-case-series/