Session Information
Date: Tuesday, November 14, 2023
Title: (2227–2256) Spondyloarthritis Including Psoriatic Arthritis – Treatment: SpA Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The EMA authorized Upadacitinib (UPA) in PsA in January 2021. UPA has shown efficacy in PsA refractory to anti-TNF in a clinical trial (RCT). Our objectives are: a) to study the effectiveness and safety of UPA in the 1st clinical practice (RWE) cases in Spain and b) to compare RWE patients with those of RCT.
Methods: Multicenter study of 134 patients with PsA treated with UPA in Spain. The diagnosis of PsA was made using CASPAR criteria. Patients with refractory PsA from 29 Rheumatology Services (January 2021-January 2023) who had received ≥1 dose of UPA (15 mg/d) with at least 1 follow-up visit were included. Refractory PsA was defined if low clinical activity or remission had not been achieved with biological (b) and/or targeted synthetic (ts) DMARDs.
The outcomes were the effectiveness, safety and saving of corticosteroids (CS). A comparative study was carried out between this RWE cohort and those of the SELECT-PsA 2 RCT.
Results are expressed as percentages, mean±SD or median [IQR] depending on the distribution of the variable.
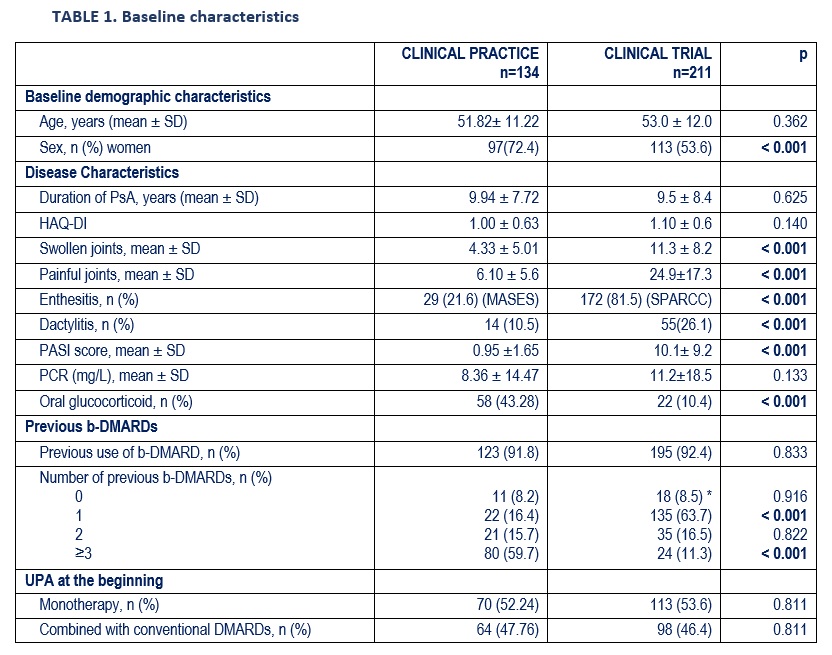
Results: 134 patients (97 women) were studied, mean age 51.8±11.2 years (Table 1). The joint pattern was: peripheral (61.9%), mixed (30.6%) and axial (7.5%). During the evolution, they had also presented enthesitis (35.3%), dactylitis (25.4%), skin involvement (73.9%) and onychopathy (24.4%).
Prior to the UPA, they had received oral CS (68.7%) (mean maximum dose of prednisone 13.4±9.3) and a mean per patient of csDMARDs (1.8±1.0) and b-DMARD (3.3±2.2). The b-DMARDs were: Adalimumab (n=101), Secukinumab (66), Etanercept (53), Ixekizumab (44), Ustekinumab (44), Certolizumab (37), Infliximab (30), Golimumab (26), Guselkumab (2), Abatacept (2), Brodalumab (1). In addition, they received the following ts-DMARDs: Tofacitinib (n=29), Apremilast (27), Filgotinib (1).
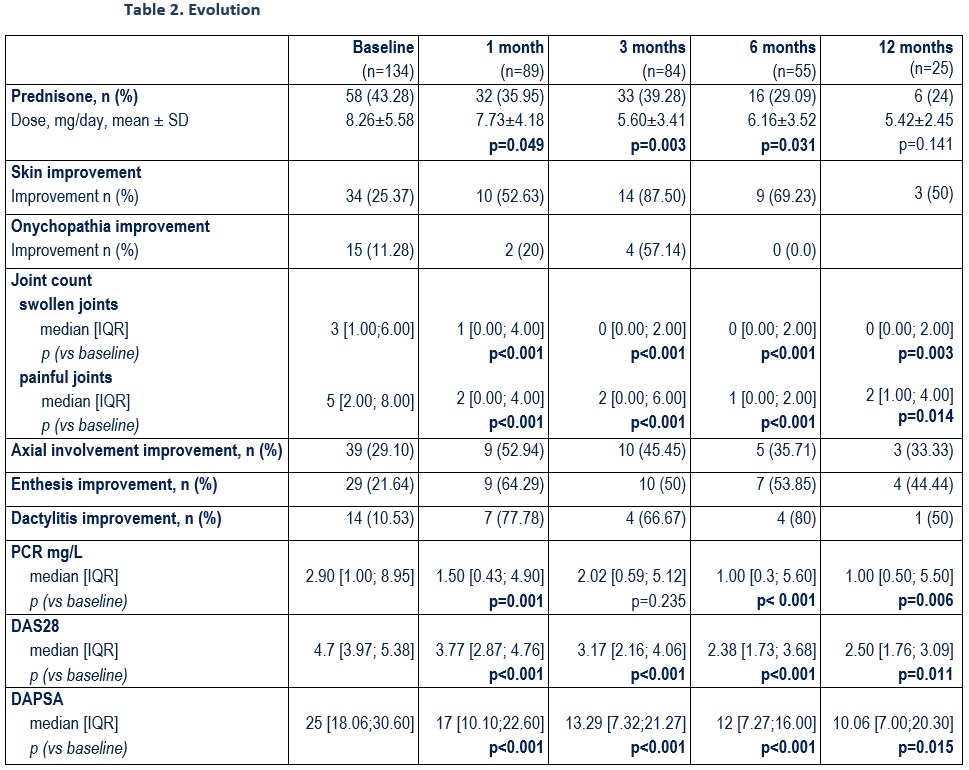
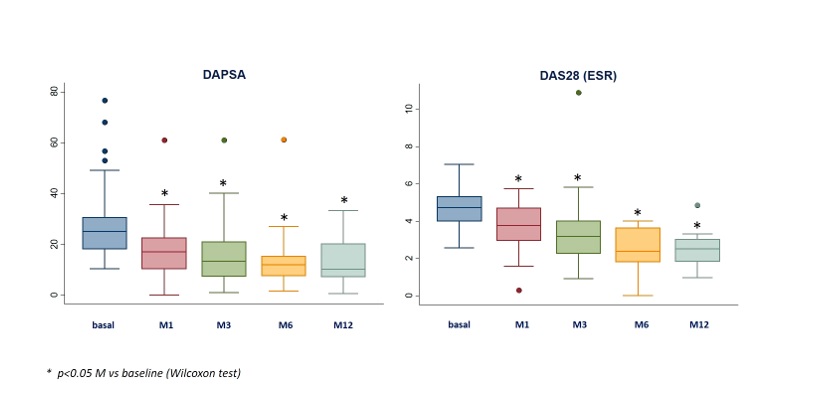
UPA at baseline was associated with: a) prednisone (43.3%; mean dose 8.3±5.6 mg/d). b) csDMARDs (n=64; 47.8%): MTX (n=39), LEF (19), SSZ (10). At the start of the UPA they presented peripheral arthritis (78.4%), axial activity (29.1%), skin involvement (25.4%), onychopathy (11.3%), enthesitis (21.6%) and dactylitis (10.5%). After a mean follow-up of 5.9±5.1 months, a rapid and sustained improvement was observed in the activity indices (table 2, figure) and in the laboratory tests (table 2).
At the 6th month, an improvement in axial involvement (35.7%) and extra-articular manifestations was observed: dactylitis (80%), enthesitis (53.8%) and skin involvement (69.2%), as well as a CS-sparing effect (p=0.031) (table 2).
The RWE patients compared to the RCT were mostly women, refractory to a greater number of previous b-DMARDs and received more concomitant CS (Table 1).
No serious adverse effects (AE) were observed. Minor AE were reported in 23 (17.2%) patients. UPA was discontinued in 44 (32.8%) (28 ineffectiveness, 4 patient decision, 4 infection, 2 de novo anterior uveitis episodes, 1 thrombosis, 1 surgery, 1 pregnancy, 1 urticaria and 1 diarrhea).
Conclusion: In this study, the first patients treated with UPA in PsA in RWE in Spain received more CS simultaneously and were refractory to a greater number of b-DMARDs than those in the RCT. As in the RCT, UPA was effective, fast, and relatively safe in refractory APs in RWE.
To cite this abstract in AMA style:
Galindez-Agirregoikoa E, Prieto-Peña D, Garcia Vivar M, Vega-Alvarez L, Vergara C, Urionaguena I, Ramos-Giráldez C, Almodovar R, Joven Ibáñez B, Garcia-Vicuna R, Jovani V, González T, Martínez-Ferrer À, Urruticoechea Arana A, Flores Robles B, Campos Fernández C, Lopez Nunez L, Belzunegui Otano J, Pavia Pascual M, Rubio E, Ramos-Calvo A, Busquets N, Pérez Gómez A, Ortiz Sanjuan F, Melero-Gonzalez R, Macía C, Puche Larrubia M, Pinto Tasende J, Fernandez C, Martinez-Vidal M, Calvo- Alén J, Beltran-Catalan E, Moreno M, Pérez-Barrio S, Gorostiza Hormaeche i, Blanco R. Upadacitinib in Refractory Psoriatic Arthritis. Multicenter Study of 134 Patients in Clinical Practice [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/upadacitinib-in-refractory-psoriatic-arthritis-multicenter-study-of-134-patients-in-clinical-practice/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/upadacitinib-in-refractory-psoriatic-arthritis-multicenter-study-of-134-patients-in-clinical-practice/