Session Information
Date: Sunday, November 8, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
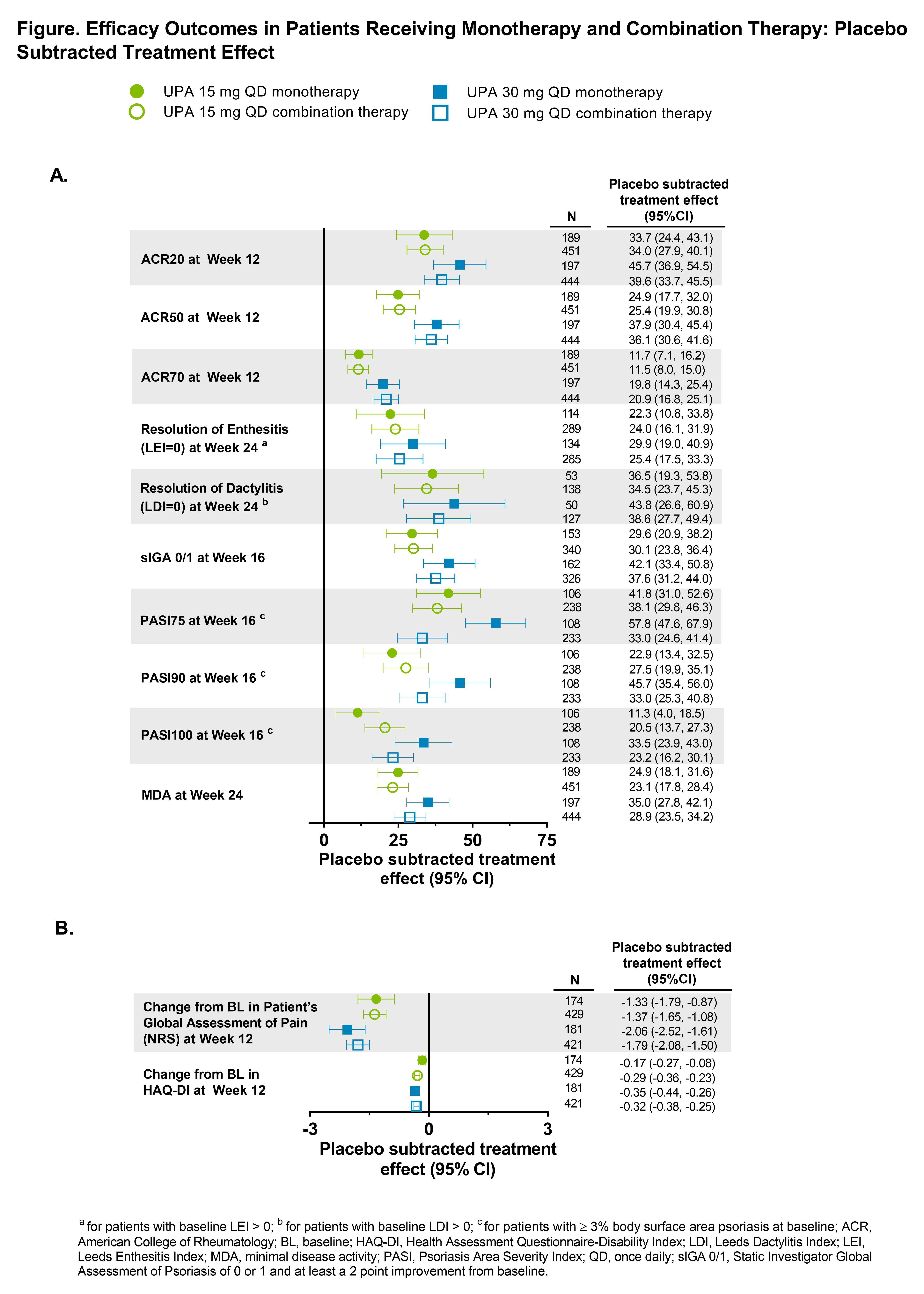
Background/Purpose: Approximately 40% of PsA patients (pts) on advanced therapy are on monotherapy.1,2 Upadacitinib (UPA) has shown efficacy and safety in pts with active PsA in the Phase 3 SELECT-PsA 1 and SELECT-PsA 2 clinical trials.3,4 This analysis assessed the efficacy and safety in the subgroups of pts who were treated with UPA as monotherapy or in combination with non-biologic disease-modifying antirheumatic drugs (non-bDMARDs).
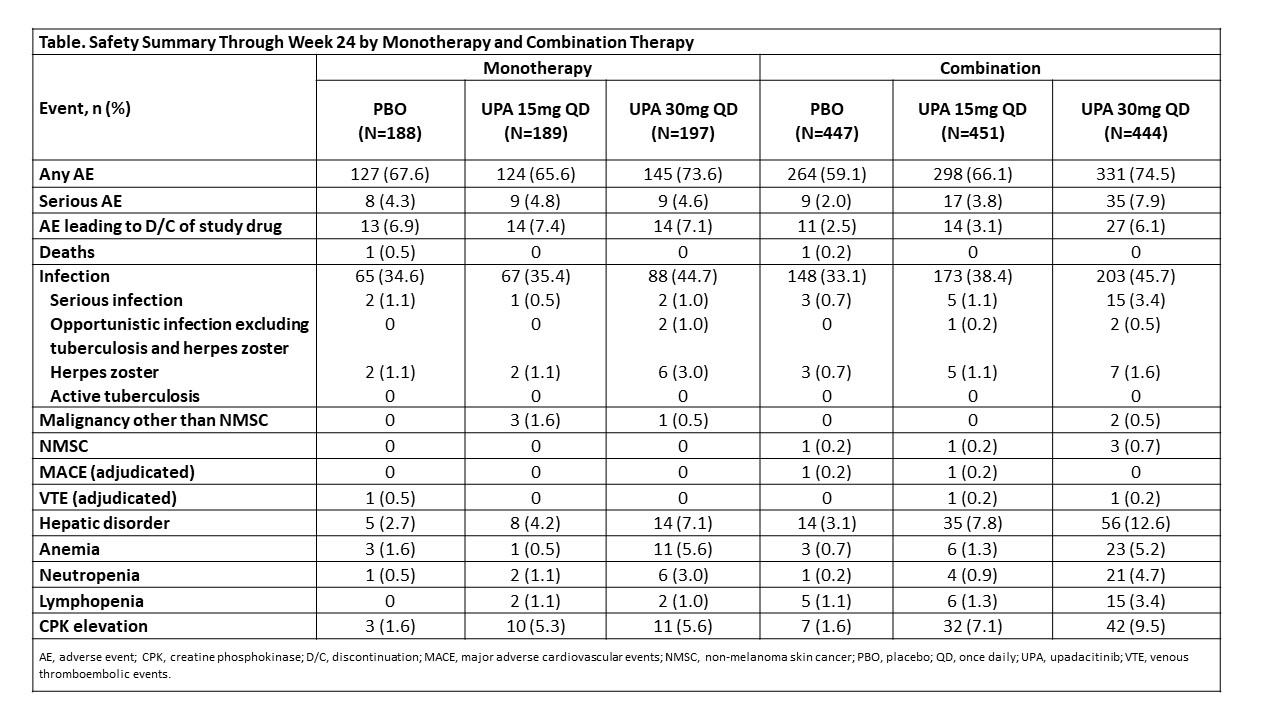
Methods: The SELECT-PsA program enrolled pts with prior inadequate response (IR) or intolerance to ≥1 non-bDMARD (N=1705) and prior IR or intolerance to ≥1 bDMARD (N=642). Data from both trials was integrated for pts receiving placebo (PBO), UPA 15 mg once daily (QD) and UPA 30 mg QD; adalimumab data was excluded from this analysis as it was only evaluated in SELECT-PsA 1. Stable background treatment of ≤2 non-bDMARDs was permitted; background therapy was not required. This analysis includes comparison of UPA monotherapy and combination therapy for the endpoints: ACR20/50/70 responses and change from baseline in pain and HAQ-DI (Week 12); Static Investigator Global Assessment of Psoriasis of 0 or 1 and at least a 2-point improvement from baseline and PASI75/90/100 responses (Week 16); proportion of pts achieving resolution of enthesitis, dactylitis, and minimal disease activity (Week 24). Binary outcomes were analyzed using the Cochran-Mantel-Haenszel-method and continuous outcomes were analyzed using mixed-effects model for repeated measures in the subgroups of UPA monotherapy and combination therapy. Point estimates and 95% confidence intervals (CIs) of the PBO subtracted treatment effect were calculated. Treatment-emergent adverse events (TEAEs) were analyzed and summarized through Week 24.
Results: Of the 1916 pts included in the analysis, 574 (30%) received monotherapy and 1342 (70%) received combination therapy; 84% in the combination therapy group received MTX +/- another non-bDMARD. Both UPA monotherapy and combination therapy led to improvements in efficacy outcomes vs PBO (Figure). Across endpoints, for each UPA dose, generally consistent point estimates of the PBO subtracted treatment effect and associated overlapping CIs were observed between pts who were on UPA monotherapy and those who were on combination therapy. Generally, the frequency of AEs, including serious AEs, were comparable with UPA when administered as monotherapy and combination therapy (Table). The frequency of AEs of serious infections and hepatic disorder were lower with monotherapy while the frequency of AEs leading to discontinuation of study drug were lower with combination therapy. Most hepatic disorders were transient transaminase elevations.
Conclusion: In the SELECT PsA Phase 3 trials, the efficacy and safety of UPA was generally consistent when administered as monotherapy or when given in combination with non-bDMARDs. Results from this analysis support the use of UPA with or without concomitant non-bDMARDs.
References
1. Ianculescu I and Weisman MH, Clin Exp Rheumatol 2015; 33:S94–S97.
2. Mease PJ, et al. RMD Open 2015; 1:e0000181.
3. McInnes IB, et al. Ann Rheum Dis, 2020; 79:12.
4. Genovese MC, et al. Ann Rheum Dis, 2020; 79:139.
To cite this abstract in AMA style:
Nash P, Richette P, Gossec L, Marchesoni A, Kato K, Blondell E, Lesser E, McCaskill R, Feng D, Anderson J, Ruderman E. Upadacitinib as Monotherapy and in Combination with Non-biologic DMARDs for the Treatment of Psoriatic Arthritis: Subgroup Analysis from Two Phase 3 Trials [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/upadacitinib-as-monotherapy-and-in-combination-with-non-biologic-dmards-for-the-treatment-of-psoriatic-arthritis-subgroup-analysis-from-two-phase-3-trials/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/upadacitinib-as-monotherapy-and-in-combination-with-non-biologic-dmards-for-the-treatment-of-psoriatic-arthritis-subgroup-analysis-from-two-phase-3-trials/