Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Due to its efficacy and perceived safety, methotrexate (MTX) has become the foundation disease-modifying drug for rheumatoid (RA) and psoriatic arthritis (PsA) however its tolerability has not been a focus of research. The objective of our study therefore was to analyse this by identifying RA and PsA patients in whom MTX was discontinued and to determine the reasons for this. We were specifically interested in how long patients remained on MTX prior to withdrawal.
Methods:
A retrospective review of our institutions electronic database was undertaken to identify all RA and PsA patients who had been prescribed MTX. Patients who had discontinued MTX were then identified from both the electronic and paper-based records and the reasons for this were categorised. The duration of MTX treatment was then assessed in those who had stopped treatment due to intolerability.
Results:
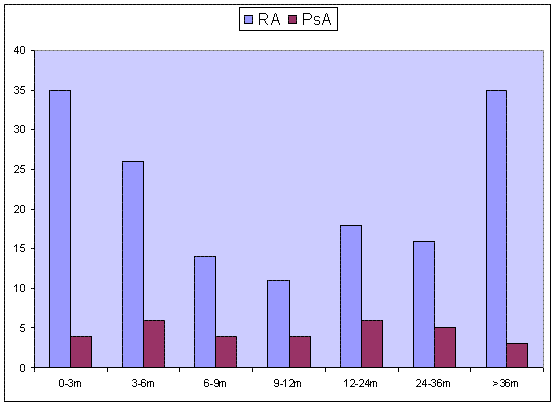
762 RA and 193 PsA patients had received MTX. In those the DMARD had been stopped in 260 (34%) RA patients and 71 (36%) PsA patients with intolerability cited as the most common reasons for this (60% and 63% in RA and PsA respectively). Haematological abnormalities (leukopenia and thrombocytopenia) were reported more frequently in RA (11.5% vs 6.8% p<0.05) and liver enzyme abnormalities more common in PsA (27% vs 12%; p<0.05). The most common symptoms resulting in MTX withdrawal were nausea, shortness of breath, feeling generally unwell, headache and fatigue. Other reasons for stopping MTX included: ineffectiveness, planned pregnancy, lifestyle choice and disease remission. The median duration of MTX treatment was 10 months in both groups, mean duration 21 and 18.6 months in RA and PsA groups respectively (Range: 0.25 – 122.25 months for RA, 1.00-79.25 months for PsA). The mode was at 3 months for RA and 1 month for PsA. See figure 1 for duration of MTX treatment.
Conclusion:
Overall one third of patients with RA and PsA stop MTX most commonly due to poor tolerability. In the context of chronic disease the median duration of treatment is short (10 months). Clinicians need to be aware of the frequency of MTX intolerability, and that this may occur early in the treatment cycle, and to alter therapy accordingly to optimise outcomes.
Figure 1. Duration of MTX therapy in patients who stopped treatment due to side effects
Disclosure:
A. Negoescu,
None;
E. Nikiphorou,
None;
A. P. Malaviya,
None;
A. Badcock,
None;
J. D. Fitzpatrick,
None;
C. T. Goudie,
None;
A. J. Ostor,
None.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/unmasking-the-tolerability-of-methotrexate-in-patients-with-rheumatoid-and-psoriatic-arthritis-a-retrospective-review-of-discontinuation-from-a-large-uk-cohort/