Session Information
Date: Sunday, October 21, 2018
Title: Rheumatoid Arthritis – Treatments Poster I: Strategy and Epidemiology
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: AWARE (Comparative and Pragmatic Study of Golimumab IV Versus Infliximab in Rheumatoid Arthritis) is an ongoing Phase 4 comparator study designed to provide a real-world assessment of intravenous golimumab (GLM) and intravenous infliximab (IFX) in patients (pts) with rheumatoid arthritis (RA). The primary objective of AWARE is to assess the incidence of infusion reactions, the concomitant use of methotrexate (MTX) is also reported. The FDA approved label for GLM states that it is indicated for the treatment of patients with moderately to severely active RA in combination with MTX; however prospectively obtained real world evidence based data on the rate of GLM use without MTX has not been reported. Here we compare patient demographics, disease characteristics, response to therapy and discontinuation of GLM treated patients with and without concomitant MTX from an interim analysis (IA) of the AWARE study.
Methods: AWARE is a prospective, noninterventional, observational, multicenter 3-year study conducted in the US. RA pts (1,200 adults) were enrolled at the time of initiating treatment with GLM or IFX. All treatment decisions including MTX utilization are made at the discretion of the treating rheumatologist. Imputations of CDAI data were not performed at this IA. Data shown are mean ± standard deviation.
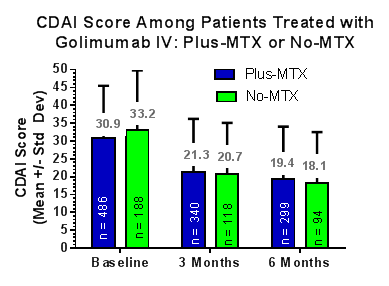
Results: 678 GLM pts were enrolled; of these 487 (71.8%) were GLM Plus-MTX and 191 (28.2%) were GLM No-MTX. Demographics are shown in the table. Response to therapy was assessed with CDAIs and shown in the figure below.
|
|
GLM Plus-MTX |
GLM No-MTX |
|
Number of Patients |
487 |
191 |
|
Age (years) |
61.7 ± 12.85 |
58.7 ± 14.55 |
|
Sex (% Female) |
87.1% |
81.7% |
|
Disease Duration (yr) |
9.0 ± 9.67 |
9.6 ± 10.74 |
|
Baseline CDAI |
30.9 ± 14.58 |
33.2 ± 16.61 |
|
Race |
|
|
|
White |
86.2% |
87.4% |
|
African American |
8.6% |
8.4% |
|
Other |
5.1% |
4.2% |
|
Weight (kg) |
83.6 ± 24.55 |
84.0 ± 22.21 |
|
BMI (kg/ m2) |
31.0 ± 8.45 |
30.8 ± 7.89 |
Overall, 92.6% of GLM Plus-MTX and 91.5% of GLM No-MTX pts had a baseline (BL) categorical CDAI disease activity of moderate or high, and 7.4% of GLM Plus-MTX and 8.5% of GLM No-MTX pts had a BL categorical CDAI disease activity of low or remission. Discontinuation from the study during the period of this IA was similar between the GLM Plus-MTX (173/487; 35.5%) and GLM No-MTX (64/191; 33.5%). 7.9% of GLM No-MTX pts reported leflunomide use.
Conclusion: At BL 28.2% of pts on GLM did not report concomitant MTX use. The demographics of the GLM Plus-MTX pts did not differ remarkably from GLM No-MTX pts. The reported early response to treatment, assessed by CDAI score after 3 months and 6 months was similar in the GLM Plus-MTX and GLM No-MTX groups. These preliminary IA data suggest that in a real-world rheumatology practice setting, use of GLM with or without concomitant MTX led to similar CDAI scores at 3 and 6 months in RA pts with predominantly moderate to high BL CDAI disease category.
To cite this abstract in AMA style:
Broadwell A, Bray V, Conaway D, Schechtman J, Kivitz AJ, Parenti D, Black S, Xu S, Langholff W, Kafka S. United States Rheumatology Practice-Based Real-World Evidence of Methotrexate Utilization and Response to Therapy in Rheumatoid Arthritis Patients Treated with Intravenous Golimumab [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/united-states-rheumatology-practice-based-real-world-evidence-of-methotrexate-utilization-and-response-to-therapy-in-rheumatoid-arthritis-patients-treated-with-intravenous-golimumab/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/united-states-rheumatology-practice-based-real-world-evidence-of-methotrexate-utilization-and-response-to-therapy-in-rheumatoid-arthritis-patients-treated-with-intravenous-golimumab/