Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: With many emerging treatments in development for lupus, clinical trial recruitment has become increasingly difficult. Growing appreciation of patient input into trial design may be of value in removing impediments to trial participation and improving the safety and well-being of participants. The current study examined treatment goals and factors influencing decisions about trial participation in patients with lupus from diverse racial backgrounds.
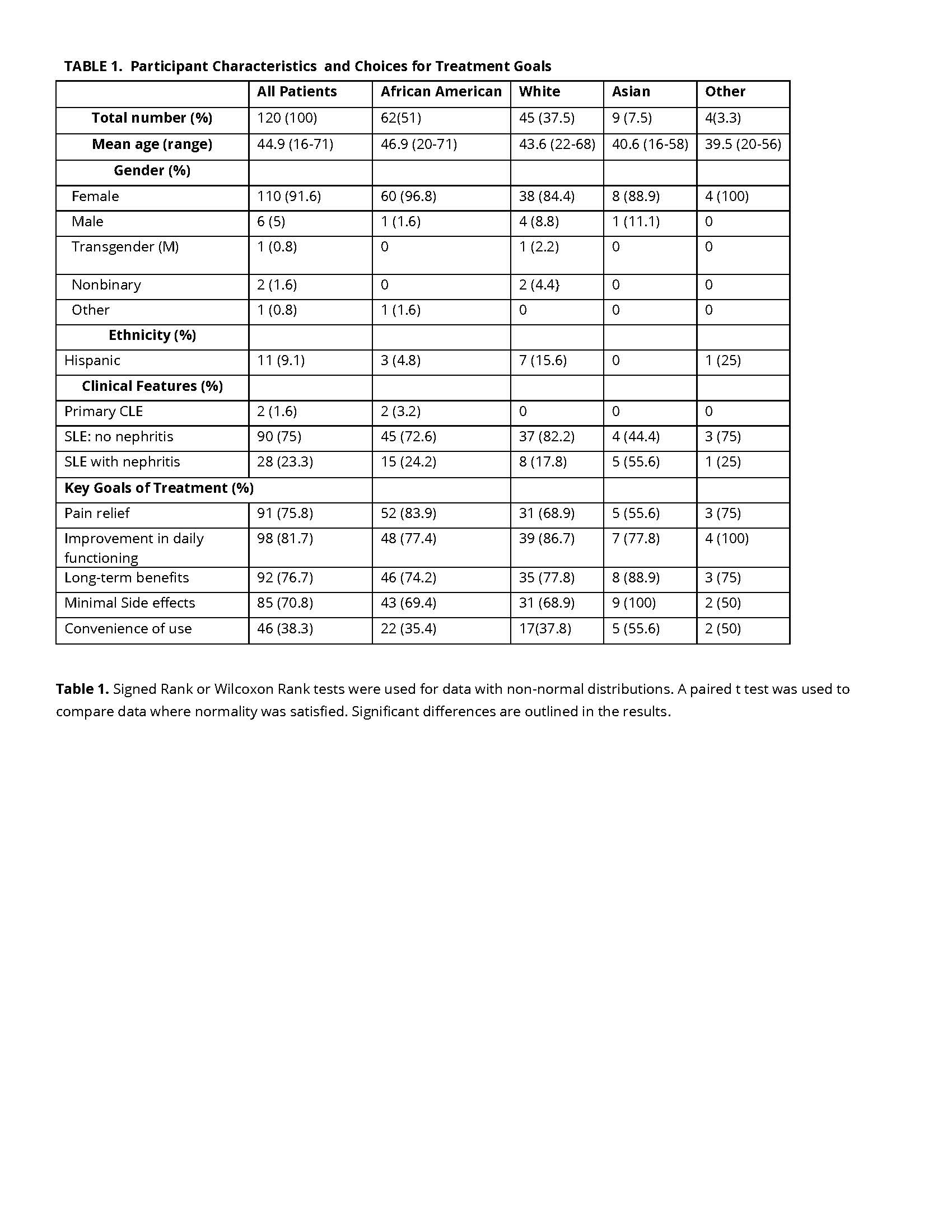
Methods: A questionnaire was developed to assess priority considerations affecting trial participation and preferences in trial designs. Delegates attending the 2024 Advocacy Summit of the Lupus Foundation of America were invited to participate. Among the delegates were 120 patients with lupus, including 62 African American, 45 White, 9 Asian, and 4 participants of other descent. The study population was predominantly female (91.6%) with mean age of 44.9 years (range 16-71), reflective of a typical trial population. There were significant variations in disease manifestations and treatment priorities among the respondents (Table 1).
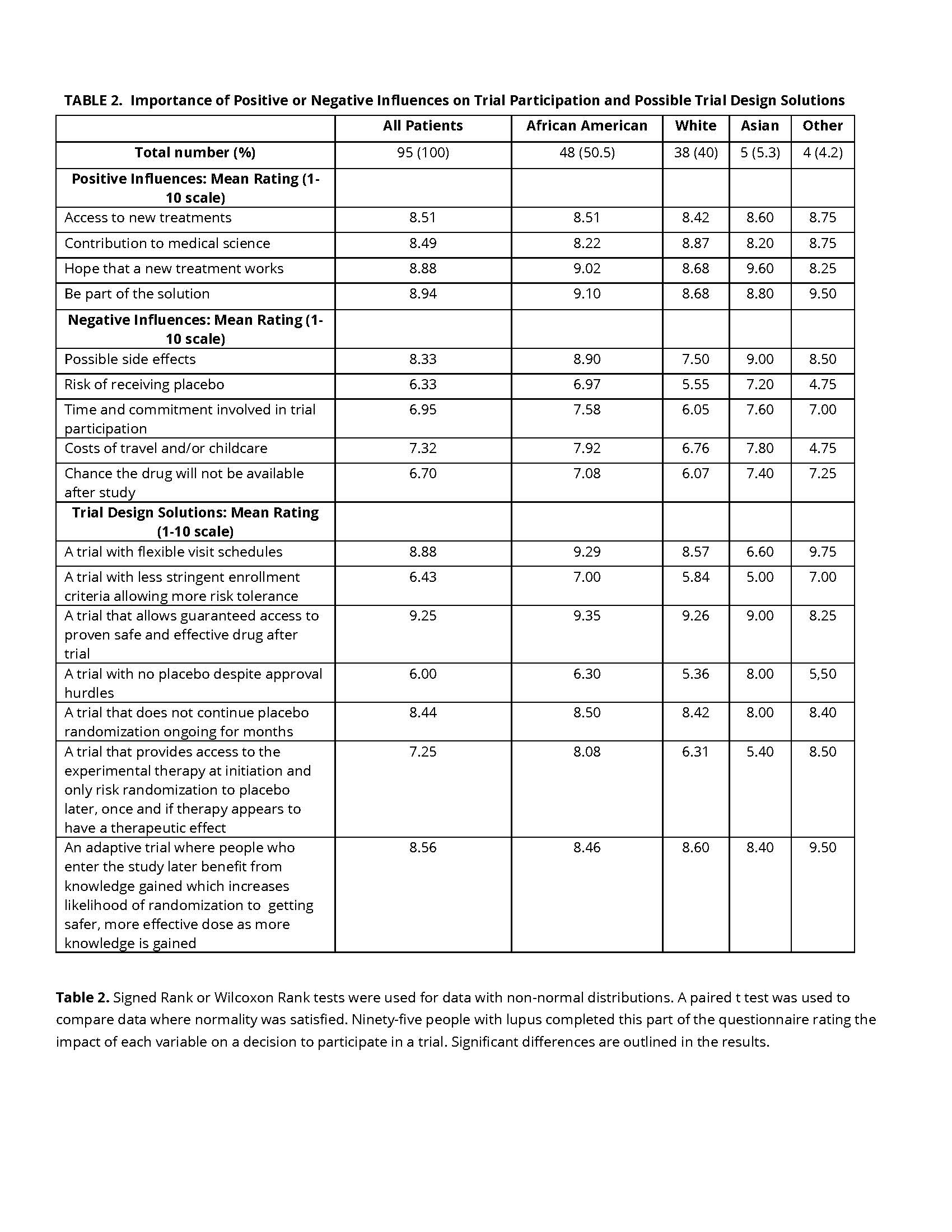
Results: Data analysis was completed using signed rank or Wilcoxon Rank tests (non-normal distributions) or paired t test where normality was satisfied. The study identified predominant goals of treatment including improved daily functioning (81.7%), and long-term benefits (76.7%). Only 38.3% of respondents chose convenience of dosing as important while 75.8% cited pain relief as a priority. African Americans and to a lesser degree White patients prioritized pain relief and daily functioning over dosing convenience (p< .001, p=.003, respectively). A subset of 95 patients reported a range of factors affecting trial participation. Positive influences on trial participation based on a 1-10 scale with 10 being the most important included access to new treatments (mean score 8.51) and contribution to medical science (mean score 8.49). Negative influences included the potential for side effects (mean score 8.33) and cost of travel/childcare (mean score 7.32). Preferences in trial design highlighted the importance of flexible visit schedules (mean score 8.88) and the concept of guaranteed access to the investigational treatment post-trial (mean score 9.25). Compared to Whites, African American patients expressed greater concern about the risk of receiving placebo when participating in a clinical trial (p=0.031) and showed preference for a trial design where all patients receive active drug early in the trial followed by randomized withdrawal that would only occur when a patient is feeling well (p= 0.002).
Conclusion: Asking patients what factors might improve their confidence in choosing to participate or increase the feasibility to do so is critical for addressing unmet needs in clinical trial recruitment. Receiving placebo as well as risk for side effects may each be impacting on willingness to participate in trials. These findings point to a need for solutions such as trials with early access to the active treatment and/or improved communications about side effect risks and risk management. Practical impediments to trial participation such as travel costs and childcare are also important and could potentially be addressed.
To cite this abstract in AMA style:
Buie J, Agyemang S, Merrill J. Understanding Treatment Goals and Factors Influencing Decisions About Clinical Trial Participation in Lupus Patients from Diverse Backgrounds [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/understanding-treatment-goals-and-factors-influencing-decisions-about-clinical-trial-participation-in-lupus-patients-from-diverse-backgrounds/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/understanding-treatment-goals-and-factors-influencing-decisions-about-clinical-trial-participation-in-lupus-patients-from-diverse-backgrounds/