Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: TNF receptor-associated periodic syndrome (TRAPS) is an autoinflammatory disease causing unprovoked fevers, myalgia, abdominal pain, rash, headaches, and, in severe cases, AA amyloidosis. It is an autosomal dominant condition resulting from variants in the TNF super family receptor 1A (TNFRSF1A) gene.1 A hallmark of TRAPS is a huge activation of the inflammatory response in the absence of autoantibodies or antigen-specific T-cells.1 Canakinumab is a high-affinity, human, selective, anti-IL-1β monoclonal antibody, developed for the treatment of autoinflammatory diseases.2 The objective of this analysis is to determine whether gene expression in whole blood can support a molecular mechanism for the activity of canakinumab in TRAPS patients.
Methods : Twenty patients with active TRAPS received open-label canakinumab 150mg sc/month for 4 months in an efficacy and safety study. These patients were assessed at Day 15 for response by physician global assessment (PGA) disease activity scale. Whole blood was collected at baseline, Day 15 and Day 113 from 19 of these patients and 1 sample each from 19 untreated age-matched healthy volunteers for analysis of gene expression levels by microarrays.
Results : All 20 patients showed improvements in PGA score (Figure 1-left). Gene expression profiles of these patients were altered by treatment with canakinumab. Forty-six differentially expressed genes showed a >2-fold change after treatment with expression levels that shifted towards that of healthy volunteers. The disease-causing gene (TNFRSF1A, Figure 1-right), drug target gene (IL-1β), and other inflammation related genes (e.g., MAPK14) were downregulated after treatment and several inflammation related pathways were evident among the differentially expressed genes. Many of the high confidence differentially expressed genes had expression levels that correlated with neutrophil count, however, neutrophil count alone could not account for the expression differences observed.
Conclusion : Altogether, the gene expression data support a model in which canakinumab increases neutrophil apoptosis and reduces pro-inflammatory signals through its inhibition of IL-1β. Canakinumab is able to reverse the overexpression of several genes associated with inflammatory response, including IL-1β. Interestingly, IL-1β blockade normalized the overexpression of the disease-causing gene, TNFRSF1A, at the RNA level, suggesting a direct impact on the main pathogenic mechanism of TRAPS.
References
1. McDermott MF, et al. Cell 1999; 97(1):133–44.
2. Lachmann HJ, et al. J Exp Med.2009; 206(5):1029–36.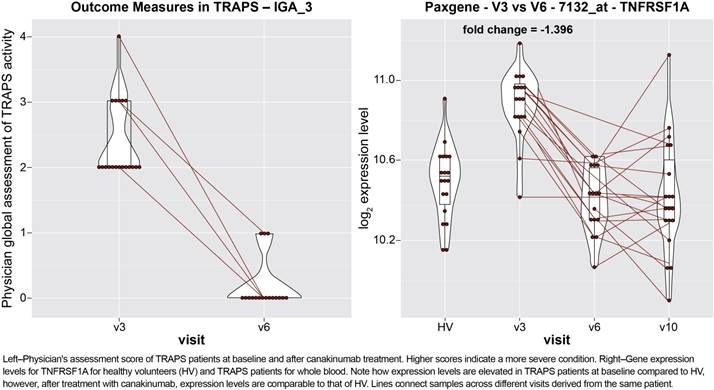
Disclosure:
R. Torene,
Novartis institute for biomedical research,
3;
M. Gattorno,
Novartis, SOBI,
2,
Novartis, SOBI,
8;
H. Lachmann,
Novartis, Celtic,
2,
Novartis.,
5;
L. Obici,
Pfizer Inc,
5,
Novartis Pharmaceutical Corporation,
8;
A. Meini,
None;
V. Tormey,
None;
R. Caorsi,
None;
L. Baeriswyl,
Novartis institute for biomedical research,
3;
U. Affentranger,
Novartis institute for biomedical research,
3;
S. Starck-Schwertz,
Novartis institute for biomedical research,
3;
M. Letzkus,
Novartis institute for biomedical research,
3;
N. Hartmann,
Novartis institute for biomedical research,
3;
K. Abrams,
Novartis Pharmaceutical Corporation,
3,
Novartis Pharmaceutical Corporation,
1;
N. Nirmala,
Novartis institute for biomedical research,
3.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/understanding-the-molecular-pathogenesis-of-and-response-to-canakinumab-treatment-in-tnf-receptor-associated-periodic-syndrome-by-gene-expression-profiling-of-whole-blood-from-patients/
