Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Despite a disproportionate burden of lupus and poorer outcomes among individuals of African descent (hereafter “black”) compared to white individuals, black individuals are underrepresented in lupus clinical trials. We aimed to explore the reasons behind this from the patient perspective using Critical Race Theory, applied to health equity research with the Public Health Critical Race Praxis (PHCRP). We hypothesized that ongoing racism and historical injustices contribute to the perpetuation of mistrust and to structural barriers within clinical trial design, which result in obstacles and fears that prevent some black individuals from participating in research.
Methods: We recruited black individuals with lupus, age >18 years old, and their caretakers (members of their support network who participate in their medical decision-making) to participate in focus groups. Participants were identified through community-based networks and organizations, lupus associations, and academic hospital networks in predominately black Boston and Chicago areas. A racially diverse, multidisciplinary study team developed our moderator guide incorporating PHCRP principles (Figure 1), and we used this framework to guide our analyses. Focus groups were transcribed verbatim. Three researchers read the transcripts and coded terms and from this, derived overarching themes, subthemes and proposed actions.
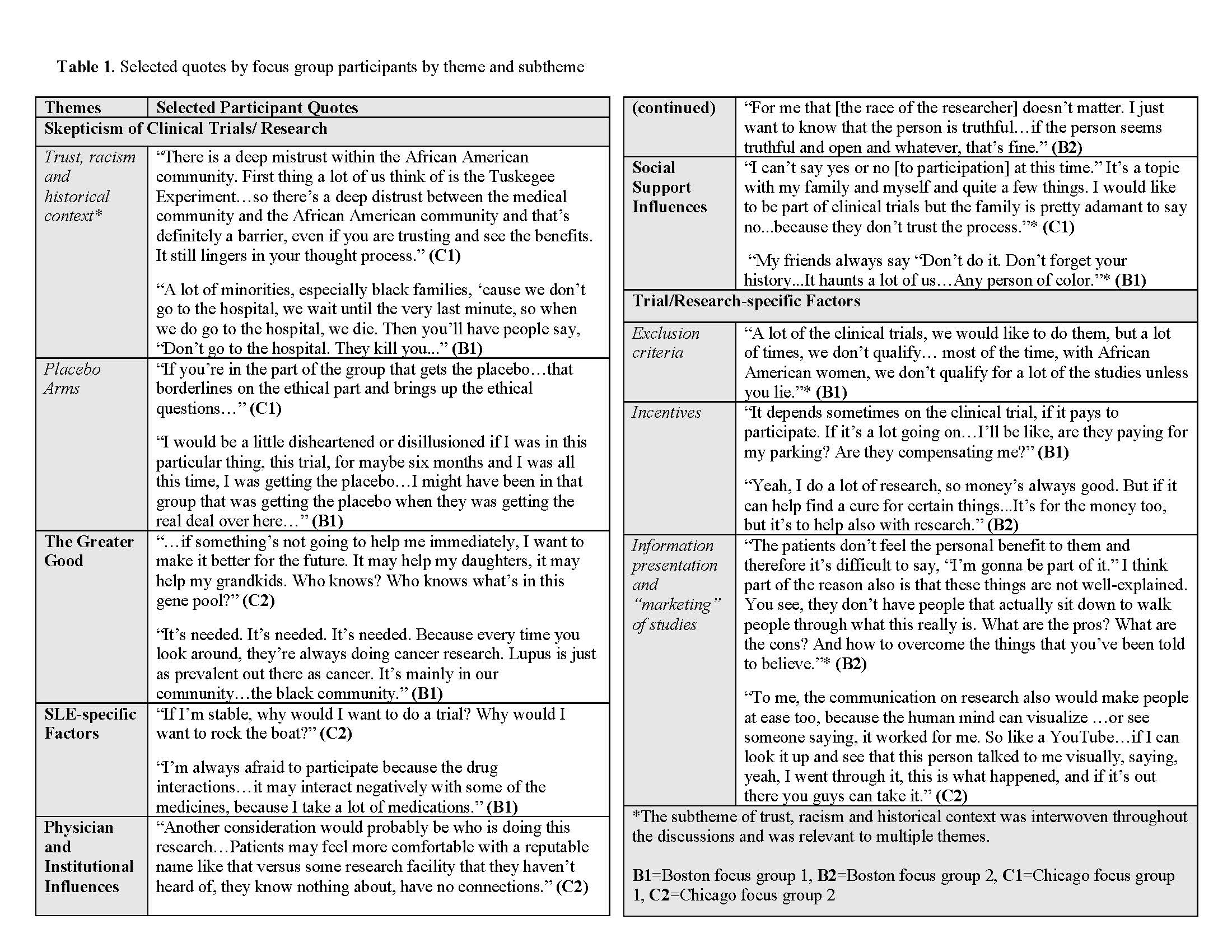
Results: We held 2 focus groups in Chicago (N=16) and two in Boston (N=15). The mean age was 54, 90% were female, and all participants identified as black. Twenty (65%) were diagnosed with SLE and 11 were caretakers. Thematic analysis of transcripts resulted in six key themes: skepticism of clinical trials/research (subthemes of trust, racism and historical context, and placebo arms), the greater good, SLE-specific factors, physician/institutional influences, social support influences, and trial/research-specific factors (subthemes of exclusion criteria, incentives and information presentation/ marketing of studies) (Table). Aspects of mistrust stemming from historical context and internalized and structural racism traversed themes. Analyses also revealed recurrent fears (e.g. being a “guinea pig”) and structural barriers (e.g. exclusion criteria), as well as mediating factors (e.g. relationships with physicians and social networks), and motivating factors (e.g. contribution to a greater good) (Figure 2). Following the PHCRP framework, actions proposed by participants included reframing the way trial information is presented and disseminated, and reevaluation of structural barriers (e.g. exclusion criteria) (Figure 1).
Conclusion: Issues of trust, racism and historical context permeated all four focus group discussions regardless of participant age. There were certain mediating factors, notably relationships between patients and their physicians, that increased the likelihood of participation in research. Our efforts to promote more inclusive, representative clinical trials should acknowledge the perpetuation of racism and the intersection of biases that occur with race, age and sex particularly in the context of lupus.
To cite this abstract in AMA style:
Feldman C, Williams J, Phillip C, Sinnette C, Mancera-Cuevas K, Canessa P, Curry G, Mason M, Ramsey-Goldman R. Understanding Clinical Trials Participation Among Individuals of African Descent with Lupus Through the Lens of Critical Race Theory: A Qualitative Analysis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/understanding-clinical-trials-participation-among-individuals-of-african-descent-with-lupus-through-the-lens-of-critical-race-theory-a-qualitative-analysis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/understanding-clinical-trials-participation-among-individuals-of-african-descent-with-lupus-through-the-lens-of-critical-race-theory-a-qualitative-analysis/