Session Information
Date: Tuesday, October 23, 2018
Title: Rheumatoid Arthritis – Treatments Poster III: Biosimilars and New Compounds
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
Biosimilar agents have changed the clinical landscape in rheumatology, gastroenterology, and dermatology. We sought to measure clinicians’ competence and knowledge of biosimilars and to address identified educational gaps related to their clinical application.
Methods:
We designed a 2-phase online educational program on biosimilars that included questions to measure participants’ knowledge and competence. These questions were administered before and then repeated after the education was delivered. Participants were also invited to submit their own questions about biosimilars and their use in clinical practice.
To identify key educational gaps, we identified questions with high incorrect responses at baseline, persistence of incorrect responses, and questions submitted by learners during Phase 1 of the education. These gaps were used to refine the teaching in Phase 2 of the education.
Results:
Between March and December 2017, a total of 1546 specialists and primary care clinicians participated in the education (35% rheumatologists, gastroenterologists, dermatologists, allergists, immunologists; 16% primary care; 48% oncologists and other specialists). Among the subset of nononcologists who provided an answer for at least 1 baseline or posteducation question, we identified persistent misunderstandings about biosimilars at baseline:
▪ 61% incorrectly believed a biosimilar could have efficacy that differs from its reference agent, a further 18% were unsure (n = 164)
– Absolute improvement in optimal response after the education was 37% over baseline (P < .0001)
▪ 62% did not understand that, in the US, biosimilars cannot be substituted at the pharmacy without the prescriber’s approval (n = 157)
– Absolute improvement in optimal response was 15% over baseline (P = .0447) after the first phase of education and 33% (P < .0001) after the second phase of education
▪ 77% did not understand extrapolation of indications among biosimilars (n = 143)
– Absolute improvement in optimal response after the education was 48% over baseline (P < .0001)
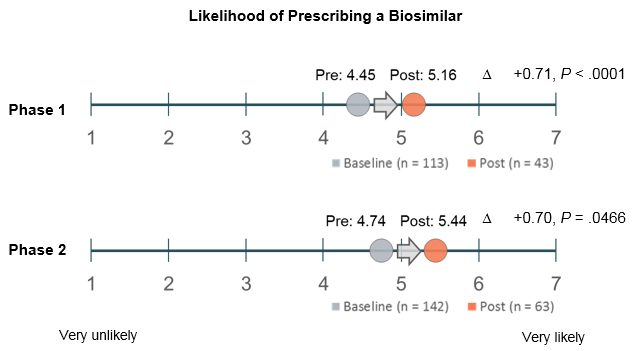
Clinicians’ willingness to prescribe biosimilars also increased incrementally after the 2 phases of education, as shown in the figure.
Conclusion:
We uncovered professional practice gaps in clinicians’ understanding of the efficacy, substitution, and indications of biosimilars, which may explain why some clinicians are reluctant to consider biosimilars as a treatment option for their patients. This educational program increased clinicians’ competence with biosimilars and their willingness to prescribe them.
To cite this abstract in AMA style:
Schwartz Z, Schulz J, Vinther A, Kuklinski A, Saag K. Uncovering Clinicians’ Gaps and Attitudes Toward Biosimilars: Impact of a 2-Phase Educational Program [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/uncovering-clinicians-gaps-and-attitudes-toward-biosimilars-impact-of-a-2-phase-educational-program/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/uncovering-clinicians-gaps-and-attitudes-toward-biosimilars-impact-of-a-2-phase-educational-program/