Session Information
Date: Tuesday, November 10, 2015
Title: Spondylarthropathies and Psoriatic Arthritis - Clinical Aspects and Treatment Poster III: Therapy
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The interleukin-17 (IL-17)
cytokine family plays a key role in the pathogenesis of psoriatic diseases of skin
and joint. Brodalumab is a fully human anti–IL-17 receptor monoclonal antibody
that blocks the activity of IL-17A, IL-17F, and IL-17A/F. Here, we evaluate the
long-term safety and efficacy of brodalumab in patients with psoriatic
arthritis (PsA) in an open-label extension (OLE) of a phase 2 study.
Methods: In a phase 2 study (NCT01516957), adults
(18–75 years) with active PsA were randomized to brodalumab (140 or 280 mg) or
placebo at weeks 0, 1, 2, 4, 6, 8, and 10. At week 12, patients could enter an
OLE and receive brodalumab 280 mg every 2 weeks (Q2W); a protocol amendment in
November 2013 resulted in dosage reduction to 210 mg Q2W for all patients.
Outcome measures based on observed data through week 108 included percentages
of patients with 20% and 50% improvement in American College of Rheumatology
criteria (ACR20 and ACR50) and changes in ACR components and Psoriasis Symptom Inventory
(PSI) score. Safety was assessed by adverse events (AEs).
Results: At baseline, patients were 64% female, 94%
white, with mean age and weight of 52 years and 91 kg, respectively. Mean PsA
duration was 9 years, 92% were rheumatoid factor negative, and mean PSI score
was 12.7. Of 168 patients randomized at baseline, 156 (52 placebo, 53
brodalumab 140 mg, and 51 brodalumab 280 mg) entered the OLE and 109 (70%)
remained at week 108.
Through week 108, 149 (96%) patients reported an AE and 23
(15%) reported a serious AE (SAE). The most frequent SAEs (n=2 each) were
coronary artery disease, cholelithiasis, and cellulitis; the most frequent AEs
(≥10% of all patients) were nasopharyngitis, upper respiratory tract
infection, psoriatic arthropathy, urinary tract infection, arthralgia,
diarrhea, sinusitis, and bronchitis. Exposure-adjusted AE rate (per 100 patient-years)
for all patients was 526; exposure-adjusted SAE rate was 14. There were no
deaths, 1 laboratory report of neutropenia, 1 case of suicidal ideation, and 11
cases of oral candidiasis.
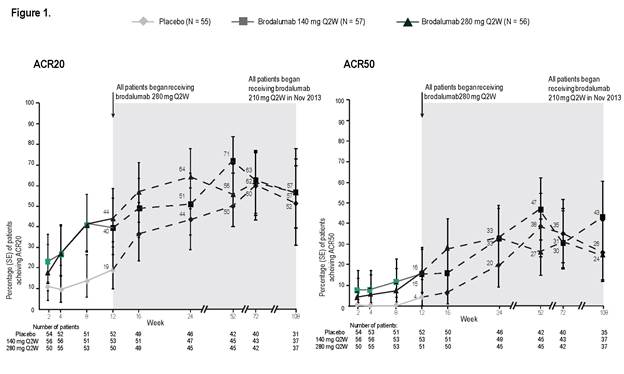
At week 12 of the study (double-blind phase), percentages of
patients with ACR20 and ACR50 (non-responder imputation [NRI] analysis) were significantly
greater in each brodalumab group than placebo: ACR20 37% (140 mg) and 39% (280
mg) vs 18% (placebo); ACR50 14% and 14% vs 4%. Through week 108 of the OLE, we
continued to observe meaningful clinical benefit in ACR20 and ACR50 (Figure 1,
as observed). Continued clinical benefit was also demonstrated by NRI analysis.
Responses for other outcome measures were also sustained from week 12 to 108 in
patients remaining in the OLE.
Conclusion: Treatment with brodalumab resulted in a safety
profile comparable to the overall safety profile of biologics approved for PsA and
meaningful clinical benefit that was maintained through week 108 in patients
with PsA in this ongoing OLE.
To cite this abstract in AMA style:
Genovese MC, Mease PJ, Greenwald M, Ritchlin CT, Beaulieu A, Deodhar AA, Newmark R, Feng J, Erondu N, Nirula A. Two-Year Clinical Response to Brodalumab, an Anti-Interleukin-17 Receptor Antibody, in Patients with Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/two-year-clinical-response-to-brodalumab-an-anti-interleukin-17-receptor-antibody-in-patients-with-psoriatic-arthritis/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/two-year-clinical-response-to-brodalumab-an-anti-interleukin-17-receptor-antibody-in-patients-with-psoriatic-arthritis/