Session Information
Date: Tuesday, November 14, 2023
Title: (2039–2060) Pediatric Rheumatology – Clinical Poster III: Potpourri
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Pediatric uveitis often requires systemic immunomodulatory therapy (IMT) to prevent sight-threatening complications. Autosomal dominant neovascular inflammatory vitreoretinopathy (ADNIV) is a rare autoimmune condition caused by variants in CAPN5, diagnosed in adulthood, and characterized by intermediate uveitis, retinal degeneration and neovascularization. It is asymptomatic in early stages but inevitably leads to permanent blindness despite treatment. Proteomic studies report elevated IL6 and VEGF in the vitreous, suggesting a role for targeted therapy to alter disease trajectory. Our aim is to present the visual outcomes of a pediatric ADNIV cohort after systemic IMT.
Methods: Cohort study of patients ≤18 years old with a genetic diagnosis of ADNIV, (+) CAPN5 variants (p.Leu244Pro), ultra-widefield fluorescein angiography (UWFA) and optical coherence tomography (OCT) imaging, and a minimum follow-up of 6 months (m) of systemic IMT. Treatment response was defined as a decrease in 1) vitreous cells on clinical examination, 2) retinal vascular leakage on UWFA, and/or 3) macular edema on OCT.
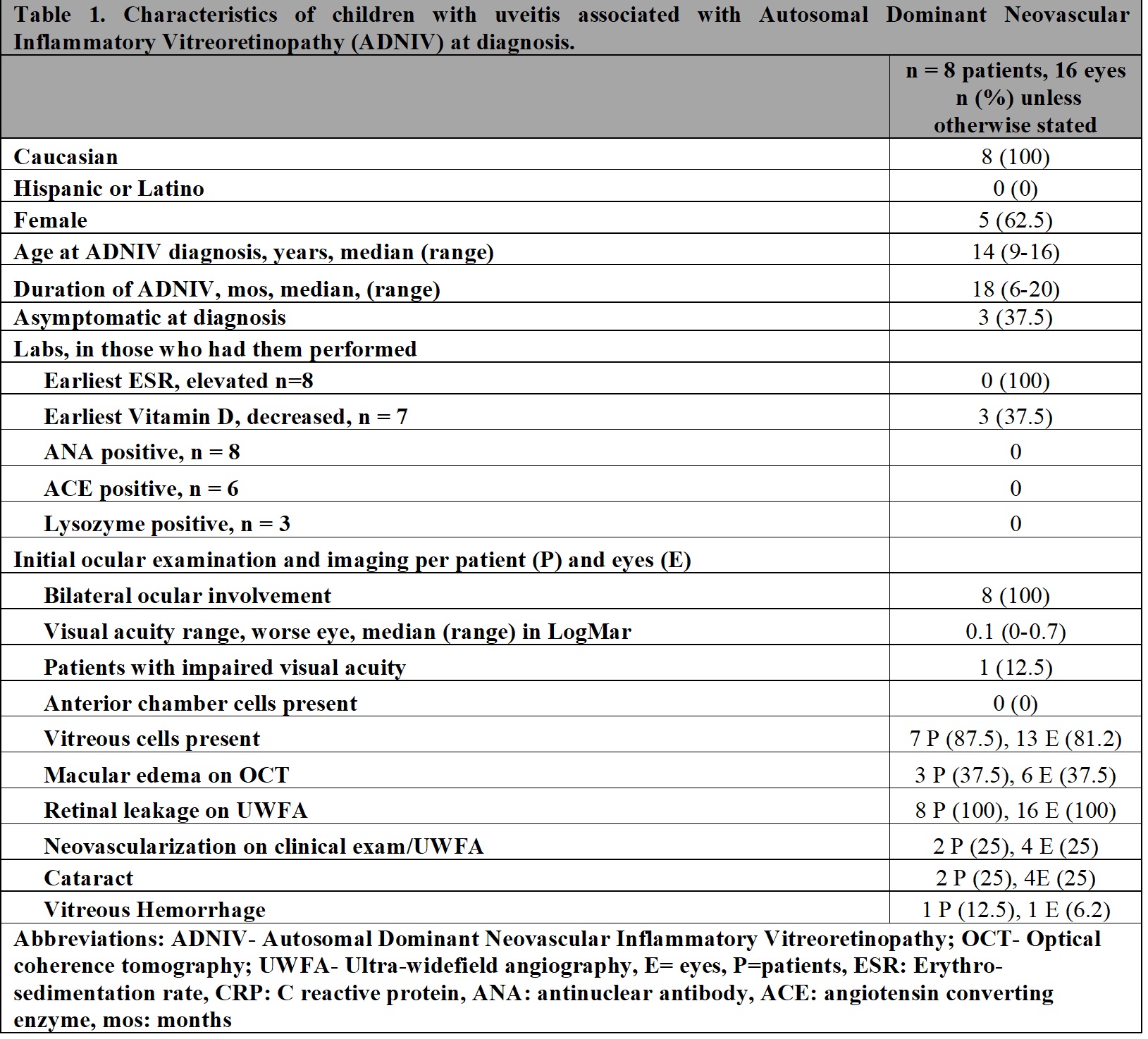
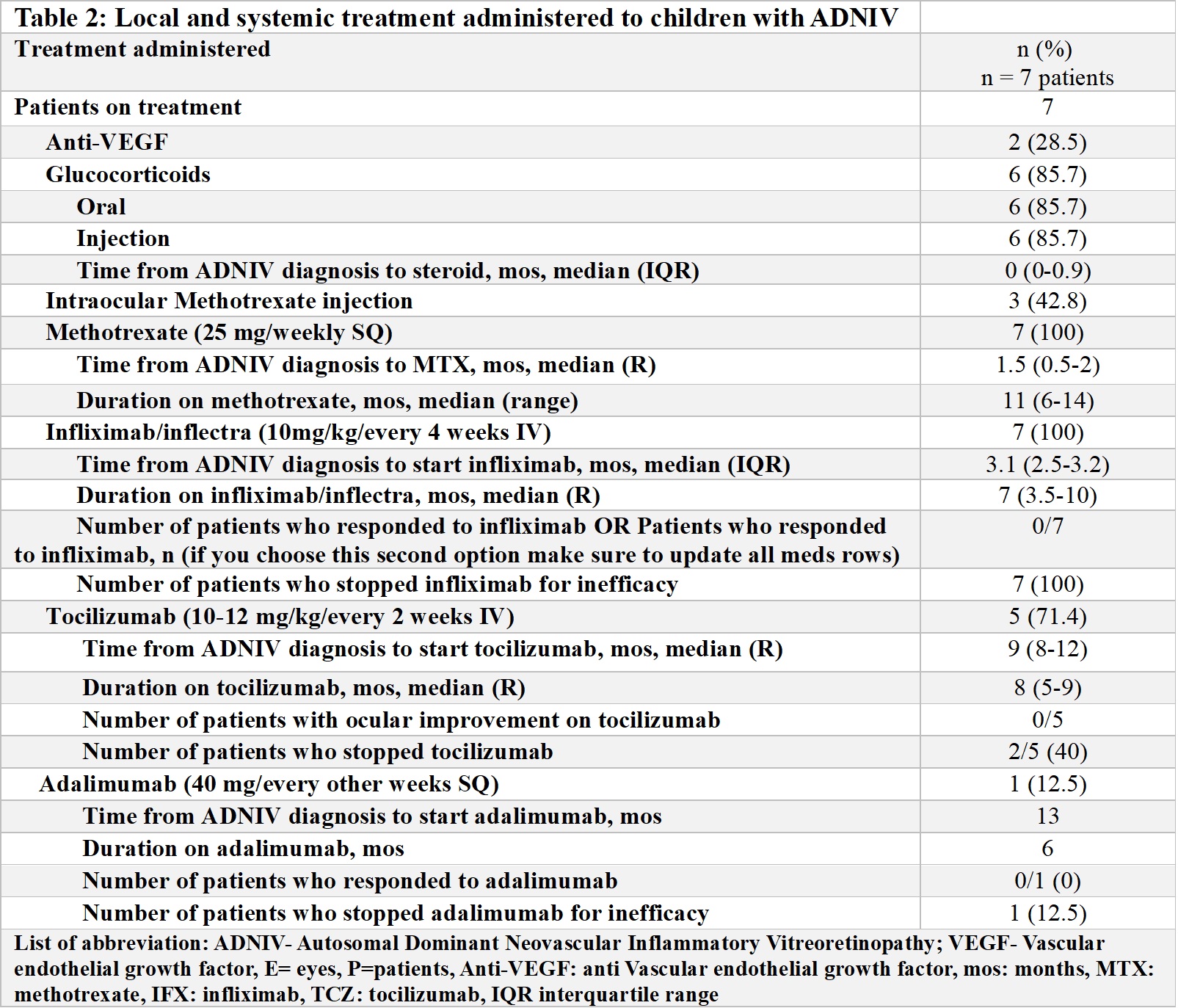
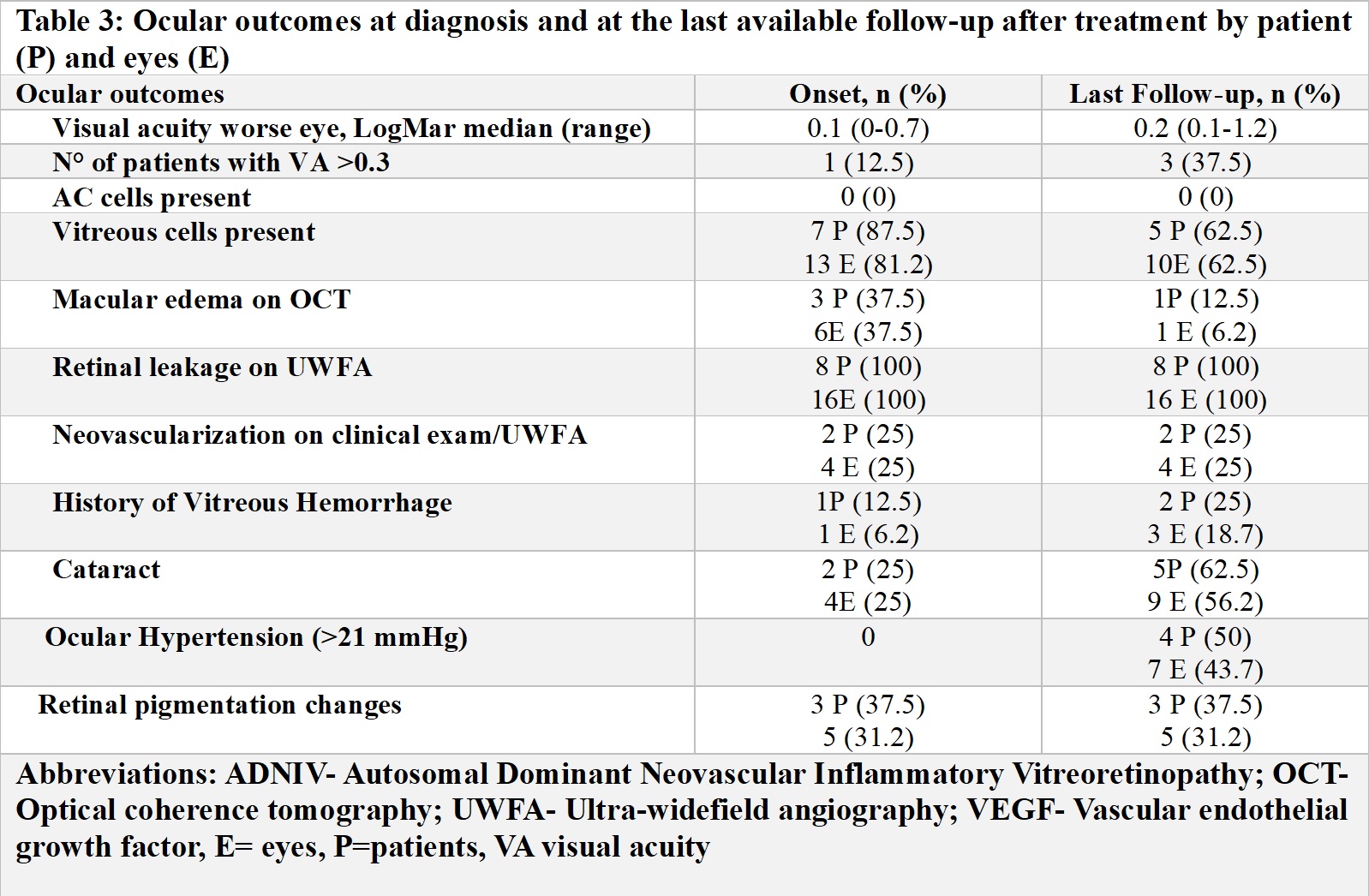
Results: 8 children (16 eyes) met inclusion criteria (Table 1). Five were female, median age at diagnosis was 14 (range [R] 9-16) years and 4 were asymptomatic. Median follow-up was 18 m (R 6-20). On initial exam, visual acuity in the worse eye was 20/100 or better, 0 had anterior uveitis, while 7 patients had vitreous cells, 8 vascular leakage (UWFA), 2 neovascularization (UWFA), 3 macular oedema (OCT) and 1 cataract. Five patients were initially treated with oral (n=5) or local/injected corticosteroids (n=4), and anti-VEGF therapy (n=2). Due to persistent inflammation, systemic IMT was started in 7/8 patients (Table 2). Methotrexate (MTX) (20 mg/weekly SQ) was the first treatment in 7 patients with the addition of infliximab (IFX) (10 mg/kg/dose every 4 weeks) after a median time from diagnosis of 3 m (R2.5-3.1) and continued for a median time of 7 m (R3.5-10). However, treatment was ineffective in all patients, and 5/7 switched to tocilizumab (TCZ) (10 mg/kg/every 2-3 weeks IV) after a median time from diagnosis of 9 m (R1-12) and 1/7 to adalimumab (ADA) (40 mg/every 2 weeks SQ) after 13 m from diagnosis. TCZ was continued for a median time of 8 m (5-9), but none of the patients showed an ocular response. Only 1/5 was able to increase the interval of administration of intraocular corticosteroids. Four/five patients discontinued TCZ for inefficacy. The single patient discontinued ADA due to inefficacy. Outcomes at the last available follow-up are reported in Table 3. Of 6 patients, 2 switched to tofacitinib and 5 received steroid implants.
Conclusion: We report the visual outcomes of the largest series of children with ADNIV treated with systemic IMT. Early testing for CAPN5 gene in at risk children, and regular screening for uveitis and vasculitis will lead to prompt intervention. MTX, IFX and TCZ were ineffective. However, we observed that TCZ was able to decrease the need of intraocular corticosteroids in one patient. Further studies are needed to determine optimal treatment in terms of molecules chosen but also of route of administration in these children.
To cite this abstract in AMA style:
Maccora I, Sood A, Schulert G, Duell A, Land P, Sapp C, Huggins J, Nguyen T, Quilan-Waters M, Sharma S, Srivastava S, Angeles-Han S. Treatment Response in a Cohort of Pediatric Patients with Autosomal Dominant Neovascular Inflammatory Vitreoretinopathy (ADNIV) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/treatment-response-in-a-cohort-of-pediatric-patients-with-autosomal-dominant-neovascular-inflammatory-vitreoretinopathy-adniv/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-response-in-a-cohort-of-pediatric-patients-with-autosomal-dominant-neovascular-inflammatory-vitreoretinopathy-adniv/