Session Information
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: A comprehensive assessment of outcome measures to assess response to treatment in ANCA-associated vasculitis (AAV) is necessary to implement.
Methods: We performed a scoping review of available randomized controlled trials (RCTs) to assess the tools previously used as outcome measures in clinical trials of AAV. Medline, Embase, Cochrane Central and ClinicalTrials.gov were searched from inception until November 26, 2018 to identify RCTs enrolling patients with granulomatosis with polyangiitis (GPA) and/or microscopic polyangiitis (MPA). This study is part of an ongoing international project to develop response criteria for AAV.
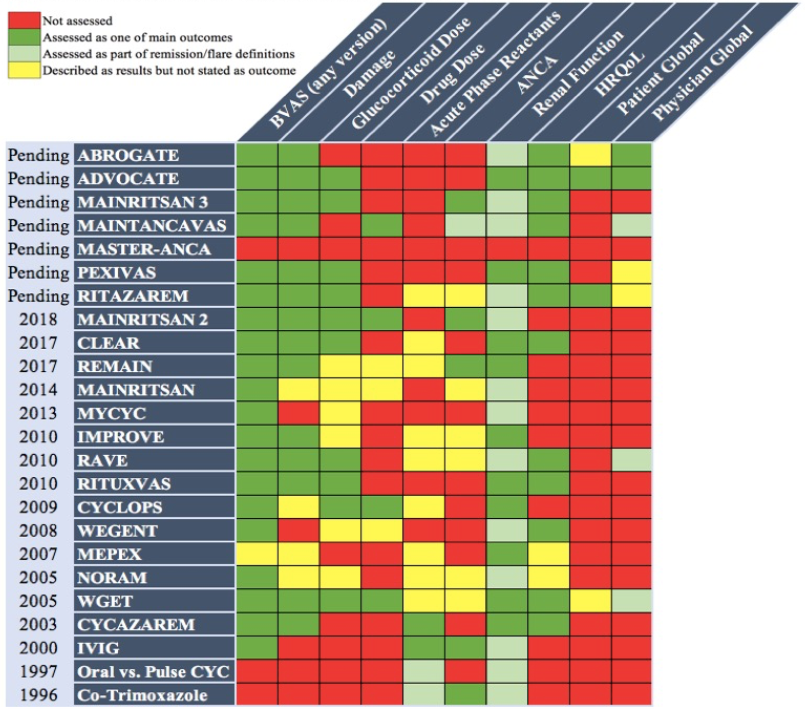
Results: Various versions of the Birmingham Vasculitis Activity Score (BVAS) were the most widely used instrument for assessing disease activity in AAV. BVAS was almost always used as a dichotomous variable (0 or > 0) providing distinction between remission and active disease. Among 24 eligible RCTs (Figure 1) reduction and/or achievement of BVAS=0 represented a main study outcome (stated as primary or secondary endpoint) in 20/24 (83%) RCTs; 6 of these trials used the BVAS/WG. Damage, mainly assessed by the Vasculitis Damage Index (VDI), was an outcome for 14/24 (58%) RCTs. Physician global assessment and patient-reported outcomes (PROs) were assessed in 7 (29%) and 14 (58%) RCTs, respectively. Assessment of renal function (part of BVAS) was a major outcome or specifically included in definitions of remission/relapse in 23/24 (96%) RCTs. Timing for outcome measure assessment differed significantly among RCTs, with baseline, 6 months (15/20 RCTs), and 12 months (14/20) after enrollment in the study being the most common time points for collecting BVAS and VDI. However, assessment of response occurred as early as 1-4 weeks after enrollment, with 6/24 (25%) RCTs assessing disease activity at 6 weeks and 13/24 (54%) at 3 months.
Conclusion: Review of outcome measures used in RCTs of AAV demonstrates the recurrent use of certain specific tools to assess disease state, although with heterogeneity in the definitions for remission/relapse, and timing of assessment. Intermediate states of disease activity of importance to patients and investigators are currently poorly defined or evaluated. Furthermore, other important outcomes in AAV, including PROs, damage measures, and global assessments are often not included as primary or confirmatory secondary outcomes in RCTs in AAV. Data-driven composite measures integrating a combination of these measures and accounting for different levels of responsiveness to therapy may result in increased power to detect clinically meaningful differences in efficacy of future treatments.
To cite this abstract in AMA style:
Monti S, Quinn K, Christensen R, Mahr A, Pagnoux C, Langford C, Jayne D, Merkel P, Tomasson G. Treatment Response Criteria for Anti-neutrophil Cytoplasmic Antibodies (ANCA)-vasculitis: Results of a Scoping Review [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/treatment-response-criteria-for-anti-neutrophil-cytoplasmic-antibodies-anca-vasculitis-results-of-a-scoping-review/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-response-criteria-for-anti-neutrophil-cytoplasmic-antibodies-anca-vasculitis-results-of-a-scoping-review/