Session Information
Date: Tuesday, November 15, 2016
Title: Spondylarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment - Poster III
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: This non-interventional, cross-sectional descriptive exploratory analysis aimed to characterize patients (pts) with PsA in the 2015 National Health and Wellness Survey (NHWS) and determine the impact of treatment, or no treatment, on pt-reported outcomes (PROs).
Methods: NHWS is a self-administered, web-based, voluntary, confidential questionnaire that used stratified randomized sampling to provide a representative sample of US adults. Respondents who reported PsA diagnosis were grouped by current treatment: advanced pharmacologic therapies (TNF inhibitors, IL-12/23 and IL-17 antagonists, PDE4 inhibitors) ± other drugs; other pharmacologic therapies (conventional synthetic DMARDs, COX2 inhibitors, NSAIDs, glucocorticoids, topical medications); or no current treatment. Short-Form 36 health survey (SF-36), Work Productivity and Activity Index (WPAI), and Patient Health Questionnaire (PHQ)-9) were investigated and summarized descriptively.
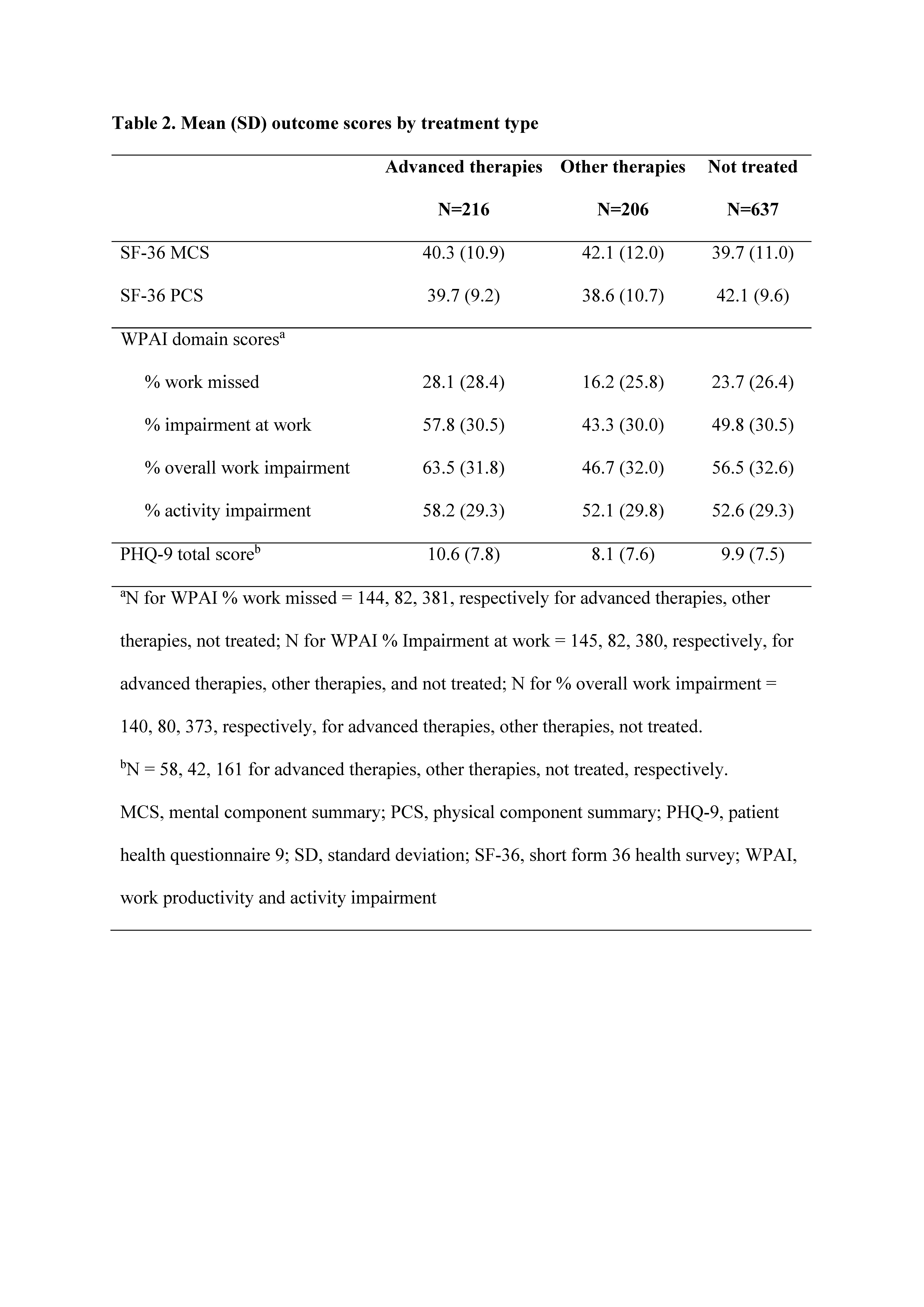
Results: Of 97,700 adults who completed the NHWS, 1059 pts reported PsA diagnosis. Of these, 216 reported treatment with advanced therapies, 206 with other therapies, and 637 reported no current treatment. Age and gender were generally balanced between treatment groups. Prior to treatment with advanced or other therapies, ~88–91% had self-reported moderate or severe PsA, and following treatment, ~54–57% reported moderate to severe PsA, which was similar to those reporting no current treatment (~58%; Table 1). SF-36 and PHQ-9 did not show wide variation in scores across treatment groups. WPAI showed that >40% of pts had work-related impairment due to PsA, despite being treated and having mild to moderate disease (Table 2).
Conclusion: We found that ~60% of pts who reported PsA diagnosis reported no current treatment. Regardless of treatment group, pts reported >20% work loss and >40% work impairment. Among pts treated with advanced and other therapies, >50% reported moderate to severe PsA, suggesting the need for additional therapies and overall better management of PsA to reduce disease activity and improve quality of life. A limitation of this analysis is that pts self-reported PsA diagnosis, and results may differ from pts having physician-reported PsA diagnosis. Further statistical analysis is needed to determine differences between groups and correlation to other health indicators. 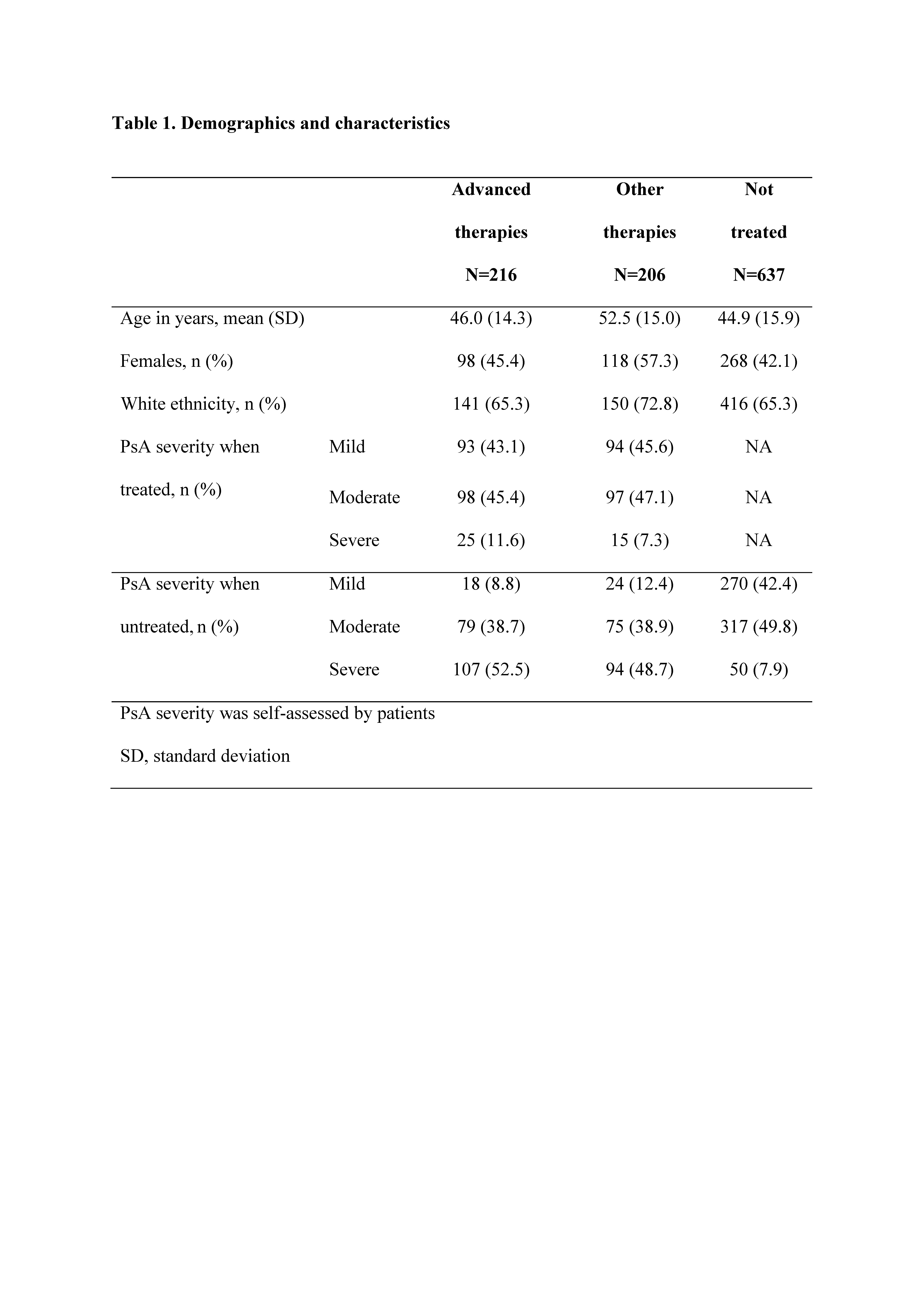
To cite this abstract in AMA style:
Gottlieb A, Gratacos-Masmitja J, Armstrong A, Lambert J, van Tubergen A, Dikranian A, Emir B, Bananis E, Smith T, Aikman L, Chen L. Treatment Patterns, Unmet Need, and Impact of Psoriatic Arthritis on Patient-Reported Outcomes in the United States [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/treatment-patterns-unmet-need-and-impact-of-psoriatic-arthritis-on-patient-reported-outcomes-in-the-united-states/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-patterns-unmet-need-and-impact-of-psoriatic-arthritis-on-patient-reported-outcomes-in-the-united-states/
