Session Information
Date: Monday, November 13, 2023
Title: (1534–1553) Vasculitis – ANCA-Associated Poster II: Epidemiology, Outcomes, & Classification
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
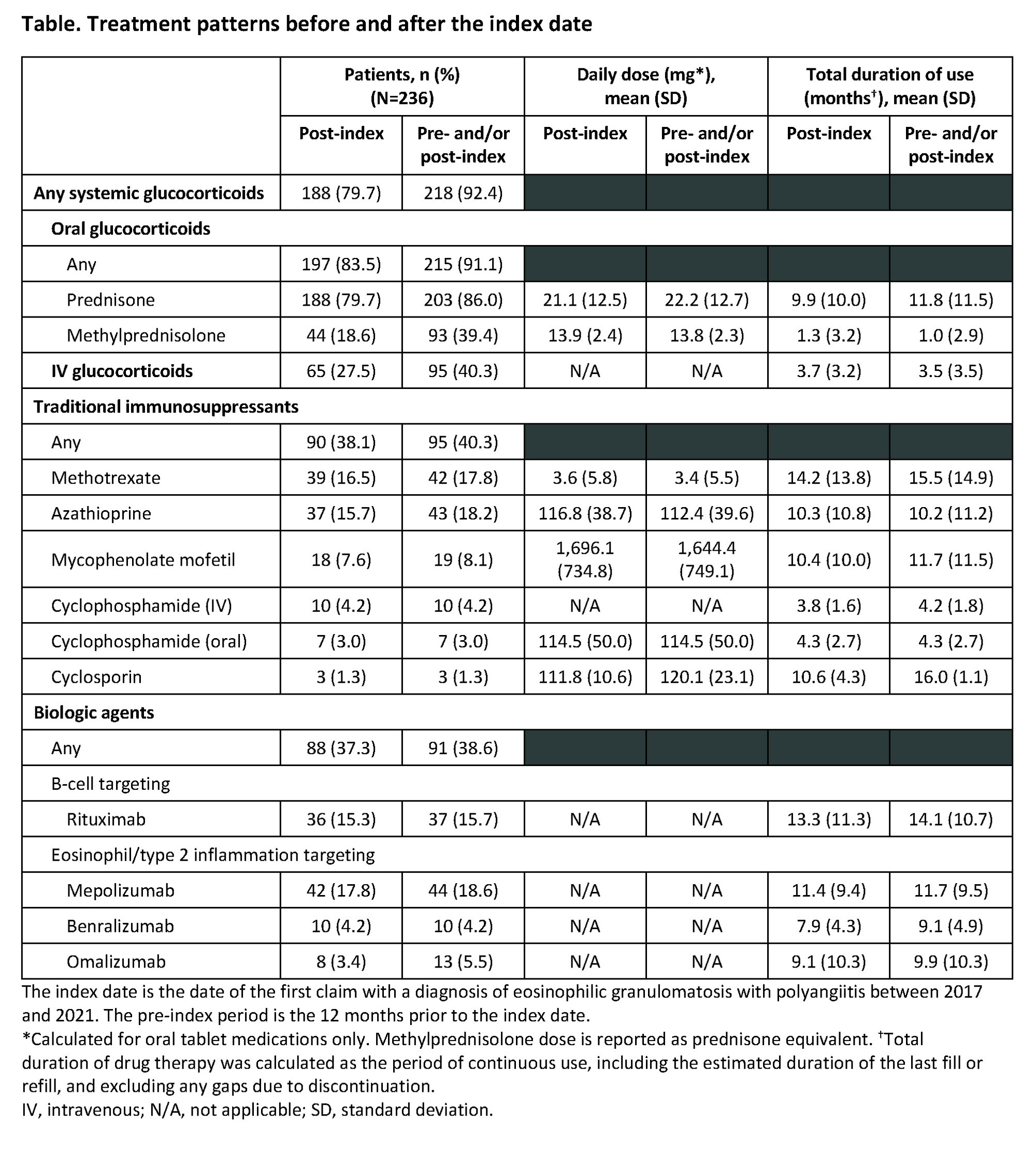
Background/Purpose: Eosinophilic granulomatosis with polyangiitis (EGPA) is a rare, immune-inflammatory disorder characterized by asthma, eosinophilia, eosinophil-rich granulomatous inflammation, and chronic necrotizing vasculitis of small-to-medium-sized blood vessels. Systemic glucocorticoids (SGCs) and immunosuppressants remain the cornerstone of treatment, even though chronic SGC use increases the risk of side effects. This retrospective analysis of US administrative health insurance claims data (Merative™ MarketScan® databases) evaluated treatment patterns for patients with EGPA.
Methods: Patients with newly diagnosed EGPA from 2017 to 2021 with ≥12 months of continuous pre-diagnosis health plan enrolment and ≥1 inpatient or ≥2 outpatient EGPA-related diagnoses (≥90 days apart, ICD-10-CM code M30.1) were included. Follow-up was from date of first observed EGPA diagnosis (index date; ID) until health plan disenrollment or database end. Use of SGCs, immunosuppressants, and biologics was analyzed, including duration and dosages (for oral medications). Duration of drug therapy was the period of continuous use including the estimated duration of last fill/refill.
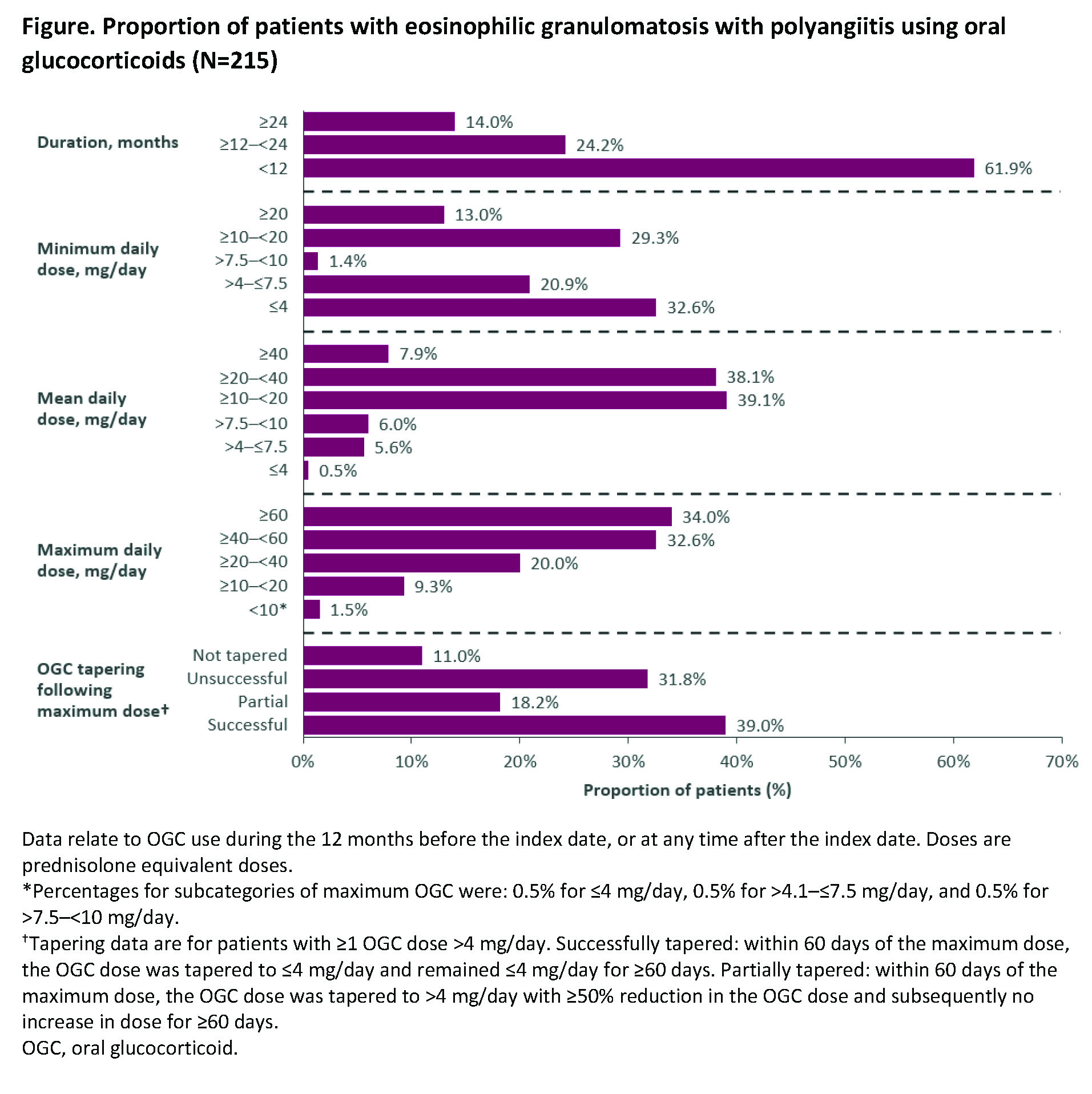
Results: 236 patients were included; 57.6% were female and mean (standard deviation [SD]) age at ID was 50.4 (14.5) years. Mean (SD) follow-up duration was 21.7 (14.6) months and 26.3% of patients had ≥30 months of follow up. In the 12 months before ID and at any time after ID, 92.4% of patients were prescribed a SGC (Table). Prednisone was used by 86.0% of patients, with a mean (SD) daily dose of 22.2 (12.7) mg and mean (SD) duration of 11.8 (11.5) months. Of the 236 patients, 91.1% received oral glucocorticoids (OGCs) either before or after ID, 14.0% had continuous use for ≥24 months, the mean (SD) average dose was 22.4 (13.7) mg/day, and 34.0% had a maximum dose ≥60 mg/day for a mean (SD) duration of 1.6 (1.6) months (Figure). Mean (SD) cumulative OGC dose was 6.3 (6.3) g with 25.6% of patients receiving ≥8 g. Among the 154 patients with at least one OGC dose >4 mg/day, OGC dose was tapered to ≤4 mg/day within 60 days of maximum dose and remained ≤4 mg/day for ≥60 days for 39.0% of patients, and OGC was tapered to >4 mg/day with ≥50% reduction in OGC dose and no subsequent increase in dose for ≥60 days for 18.2%; 11.0% did not attempt to taper their dose. Immunosuppressants were used by 40.3% of patients, most commonly azathioprine (18.2%), methotrexate (17.8%), and mycophenolate mofetil (8.1%). Mean (SD) daily dose and treatment duration were 112.4 (39.6) mg and 10.2 (11.2) months for azathioprine, 3.4 (5.5) mg and 15.5 (14.9) months for methotrexate, and 1,644.4 (749.1) mg and 11.7 (11.5) months for mycophenolate mofetil. Biologics were used by 38.6% of patients, with 15.7% using a B-cell targeting therapy (rituximab).
Conclusion: Despite the use of traditional immunosuppressants and biologics by more than one-third of patients with EGPA, the vast majority were prescribed glucocorticoids, with many receiving OGCs for long, continuous periods, and many with a maximum dose ≥60 mg/day. Most patients were unable to maintain reduced OGC doses during the follow-up period suggesting there is a need for better OGC-sparing therapies.
To cite this abstract in AMA style:
Dolin P, Kielar D, Shavit A, Keogh K, Rowell J, Edmonds C, Meyers J, Esterberg E, Nham T, Chen S. Treatment Patterns for Eosinophilic Granulomatosis with Polyangiitis (EGPA): A Retrospective Analysis of US Health Insurance Claims Data [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/treatment-patterns-for-eosinophilic-granulomatosis-with-polyangiitis-egpa-a-retrospective-analysis-of-us-health-insurance-claims-data/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-patterns-for-eosinophilic-granulomatosis-with-polyangiitis-egpa-a-retrospective-analysis-of-us-health-insurance-claims-data/