Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Eosinophilic granulomatosis with polyangiitis (EGPA) is a rare vasculitis disorder characterized by asthma, eosinophilia and the presence of eosinophilic inflammation. Patients with EGPA are mainly treated with oral glucocorticoids (OGCs), in addition to immunosuppressants and biologics. We assessed the treatment outcomes in patients with EGPA using a retrospective analysis of US administrative health insurance claims data (Merative™ MarketScan® databases).
Methods: Patients with newly diagnosed EGPA from 2017 to 2021 with ≥12 months of continuous pre-diagnosis health plan enrollment and ≥1 inpatient or ≥2 outpatient EGPA diagnosis records (≥90 days apart, ICD-10-CM code M30.1) were included. Follow-up was from EGPA diagnosis (index date; ID) until health plan disenrollment or database end. Remission (defined as no new EGPA events or hospitalization and OGC ≤4 mg/day) was reported at ID and at 6 and 12 months after ID; transition probabilities between disease activity states were reported at ID and at 6 months after ID.
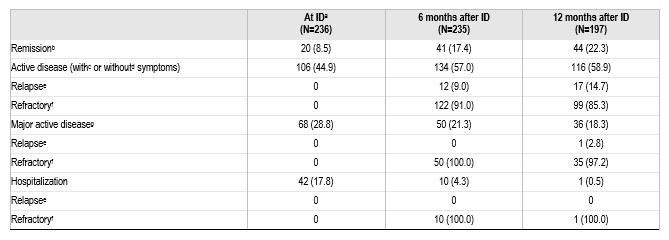
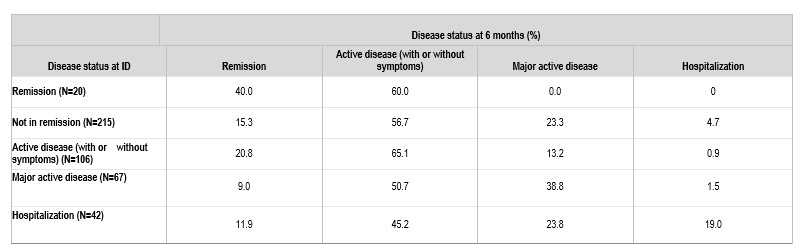
Results: A total of 236 patients with EGPA were included in the analysis; mean (standard deviation [SD]) age was 50.4 (14.5) years; 88% of patients were < 65 years old at diagnosis, and 57.6% were women. Mean (SD) duration of follow-up was 21.7 (14.6) months. Any time after ID, 197 (83.5%) patients were treated with OGCs, 90 (38.1%) with immunosuppressants (azathioprine, mycophenolate mofetil, cyclophosphamide, cyclophosphamide, cyclosporin, methotrexate), 36 (15.3%) with rituximab, 42 (17.8%) with mepolizumab, 8 (3.4%) with omalizumab, 10 (4.2%) with benralizumab, and 22 (9.3%) patients were not treated with any of the above glucocorticoid, immunosuppressants or biologics. Remission was achieved by 20 patients (8.5%) at ID, and by 41 (17.4%) and 44 (22.3%) patients at 6 months and 12 months follow-up, respectively (Table 1). Among patients in remission at ID, only 40% remained in remission at 6 months, and of those not in remission at ID, only 15% were able to achieve remission at 6 months (Table 2). Considering therapies used at ID, the remission rate 6 months later was 12.3%, 16.7%, 30.0% and 19.8% in those treated with OGCs-only, immunosuppressant ± glucocorticoid, and biologic (mepolizumab, omalizumab, benralizumab, rituximab) ± immunosuppressant/glucocorticoid, and in those receiving none of the above glucocorticoid/immunosuppressants/biologics, respectively.
Conclusion: An overall increase in EGPA remission was observed with time since diagnosis. However, the probabilities of transition between disease activity states demonstrated that current EGPA treatments have limitations in inducing and maintaining remission. These findings emphasize the need for new and more effective therapies. A limitation of the study was the inability to separately analyse remission with anti-IL-5 therapies due to small sample size.
a6 months before or at ID; bNo new EGPA events or hospitalization and OGC ≤4mg/day; cMinor EGPA ≥1 events limited to 1 organ system, with an associated change in therapy and no hospitalization; dNo new EGPA events or hospitalization and OGC ≥4mg/day; eTransition from remission at previous assessment timepoint to active disease (minor or major) at current assessment timepoint; fPresence of active disease (minor or major) in previous and current assessment timepoints; gMajor existing event, or minor existing events in 2+ organ systems, with an associated change in therapy and no hospitalization.
Data are n (%); ID, index date.
EGPA, eosinophilic granulomatosis with polyangiitis; ID, index date; OGC, oral glucocorticoid.
ID, index date.
To cite this abstract in AMA style:
Dolin P, Shavit A, Keogh K, Rowell J, Edmonds C, Kielar D, Meyers J, Esterberg E, Nham T, Chen S. Treatment Outcomes of Eosinophilic Granulomatosis with Polyangiitis (EGPA):A Retrospective Analysis of US Health Insurance Claims Data [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/treatment-outcomes-of-eosinophilic-granulomatosis-with-polyangiitis-egpaa-retrospective-analysis-of-us-health-insurance-claims-data/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-outcomes-of-eosinophilic-granulomatosis-with-polyangiitis-egpaa-retrospective-analysis-of-us-health-insurance-claims-data/