Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: To evaluate the efficacy and safety of leflunomide (LEF) for active Chinese Takayasu Arteritis (TA) patients in induction therapy compared with cyclophosphamide (CYC) and in long-term treatment with comparison between new-onset patients and refractory patients who were ineffective or recurrent after CYC treatment. The mechanism of LEF on improving the inflammatory fibrosis in TA by regulating M2 macrophages were also explored.
Methods: Based on the East China TA cohort, a total of 131 patients with active TA who were treated with LEF or CYC were enrolled. The patients were all followed up for 6 months. Their epidemiological data, clinical performance, laboratory data and imaging data at baseline and follow-up were collected for comparison of the efficacy and safety of LEF and CYC. Multivariate analysis was used to compare the response rate of the two groups of patients after 6 months of treatment. Propensity-score matching (PSM) was used to correct the baseline data of the two groups. Fifty-six of patients treated with LEF were further analyzed for their response to treatment after 6 months and 12 months. Among them, 41 patients were treated with LEF since initial visit, and 15 patients were replaced with leflunomide after treatment with CYC. Peripheral blood monocytes of sixteen TA patients were treated with LEF. The ratio of M1/M2 and the apoptosis of M2 were detected by Flow cytometry. The supernatant level of cytokines and chemokines secreted by M2a macrophages were detected by ELISA. The relative expression of profibrotic cytokines mRNA in M2a were detected by real-time PCR. Western Blot was used to measure the phosphorylation of Stat6.
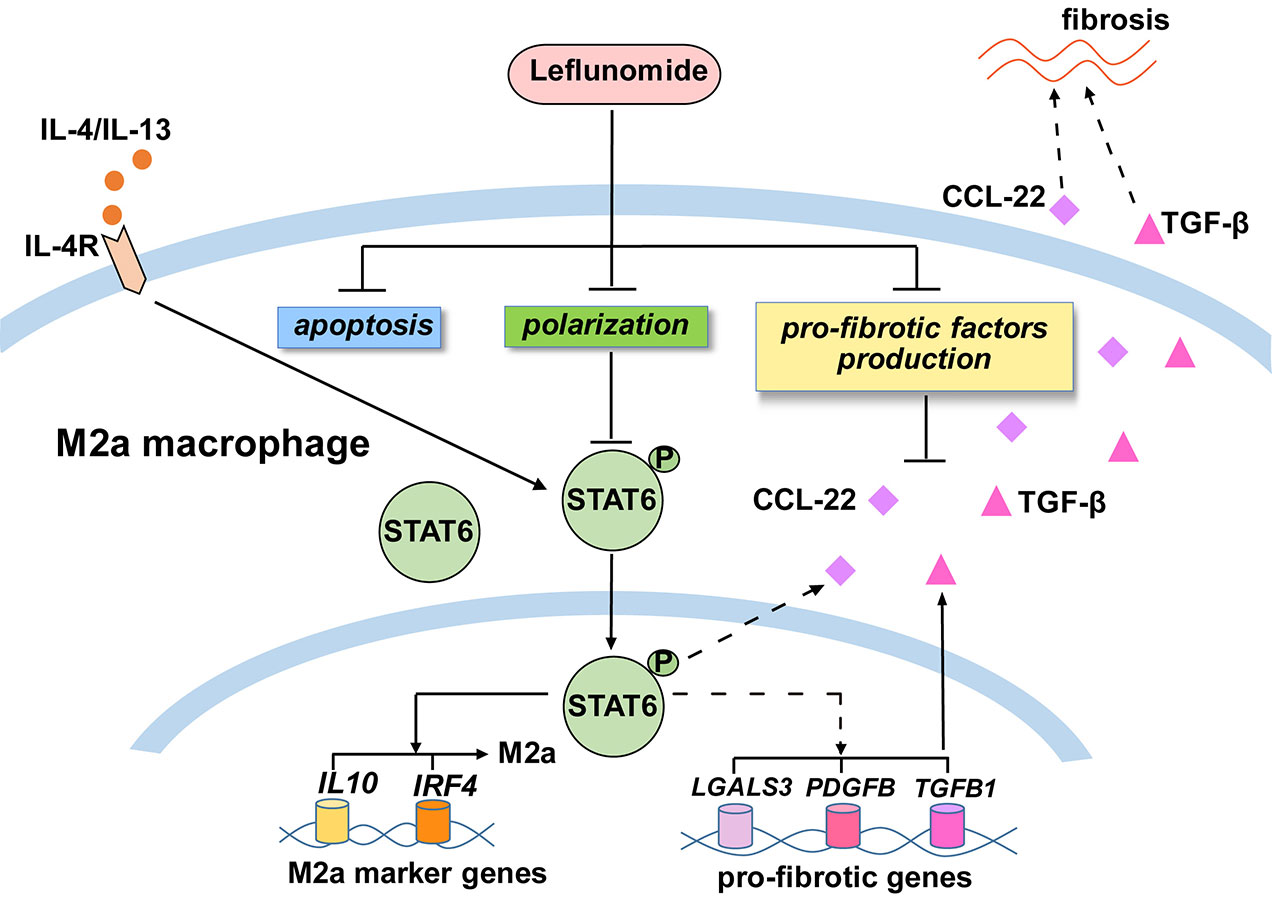
Results: Patients in the LEF group were younger and had higher erythrocyte sedimentation rate (ESR) compared with CYC group. After 6 months of treatment, the clinical remission rate of LEF group was not inferior to CYC group. The overall clinical remission rate of patients treatment with LEF was 67.9% after 6 months of treatment, and 55.4% after 12 months. The level of ESR, C-reactive protein (CRP) and NIH scores decreased significantly after 12 months of LEF treatment. Patients who reacted as resistance to CYC achieved clinical remission in more than half of them after 6 and 12 months of LEF therapy. In addition, during the 12-month follow-up, most patients treated with LEF had well drug tolerance and a low incidence of adverse reactions. LEF could inhibite M2 polarization by curtailing STAT6 phosphorylation. LEF could also promote apoptosis of M2 and reduce the release of M2-derived CCL22 as well as the expression of profibrotic cytokines including CCL22 and TGF-β in M2.
Conclusion: LEF has comparable efficacy to CYC for induction of remission in active TA. Treatment with LEF in TA can achieve clinical remission in a short period of time, and maintain long-term stability of the disease, especially for refractory patients. LEF presents safety for women of childbearing age, and can be used for long-term treatment of patients with TA. LEF could reduce vascular fibrosis potential by down-regulating number and function of M2, which, eventually, could alleviate inflammatory fibrosis of aortic lesions in TA patients.
To cite this abstract in AMA style:
Cui X, Dai X, Kong X, Chen R, Ma L, Sun Y, Jiang L. Treatment Efficacy Evaluation of Leflunomide by Regulating Macrophages in Takayasu Arteritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/treatment-efficacy-evaluation-of-leflunomide-by-regulating-macrophages-in-takayasu-arteritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-efficacy-evaluation-of-leflunomide-by-regulating-macrophages-in-takayasu-arteritis/