Session Information
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Prior authorizations (PA) are commonly required by health payers as cost-containment strategies for expensive medications, including infused biologics. In rheumatology, these medications are frequently prescribed to treat rare conditions that can be organ- or life-threatening and for which few or no medications have regulatory approval because of their relative scarcity. Infused medications often have neither generic substitutes nor oral or subcutaneous formulations and inevitably require advanced planning to administer. There are scarce data about the effect of PA requirements for infused medications on patient-oriented outcomes.
Methods: We included subjects for whom an infusible medication was prescribed in our academic rheumatology practice for a rheumatologic condition. The exposures of interest were a PA requirement and the PA outcome (approved vs. denied). The primary outcome was the difference in days from medication request to infusion. Secondary outcomes included time to PA approval, the proportion of denied PAs, and glucocorticoid exposure among those approved and denied following PA request.
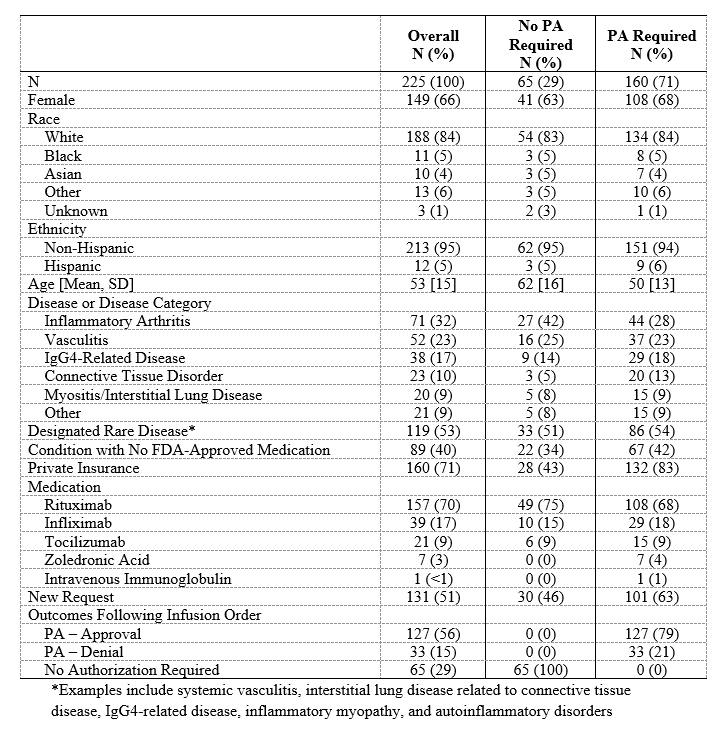
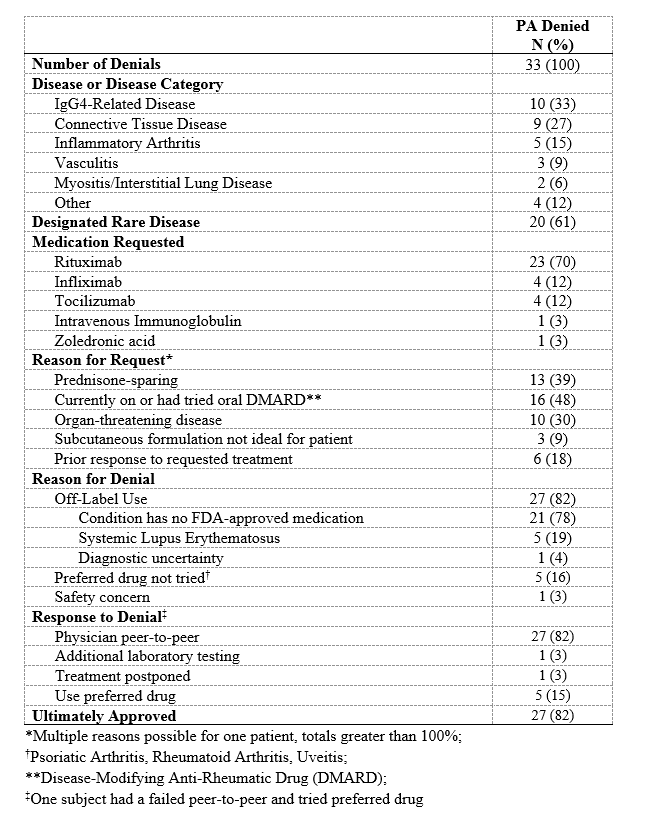
Results: Of the 225 subjects (Table 1), 160 (71%) required a PA. Of the PA-requiring cases, the conditions most often being treated were inflammatory arthritis (44, 28%) and vasculitis (37, 23%). In 67 (42%) cases requiring a PA, the condition had no FDA-approved medication. The PA request was initially denied in 33 (21%) subjects, 21 (64%) of whom had conditions with no FDA-approved medications (Table 2); twenty-seven (82%) of these denials were eventually approved. Thus, 96% of all PAs were ultimately approved. PAs were associated with a greater median number of days to infusion compared to cases in which no authorization was required (31 days [15, 60] vs 27 days [13, 41], p=0.045; Table 3). Compared to those in whom no PA was required, subjects in whom a PA request was initially denied had substantially longer time to approval (22 days [15, 41] vs 0 days [0, 0]), P< 0.001) and infusion (50 days [31, 76] vs 27 days [13, 41], P< 0.001). PA denials were associated with a 3.8-fold greater median prednisone-equivalent glucocorticoid exposure in the 3 months following the request than when a PA was not required (605mg [0, 1575] vs 160mg [0, 675], P=0.01).
Conclusion: PA requirements for infusible medications are associated with delays in treatment. There is a striking three-week delay to approval and one-month delay to treatment among those whose PAs are initially denied. PA denials are further associated with excess glucocorticoid exposure. Thus, PAs constitute a barrier to the introduction of effective treatment in an expeditious manner. Because the great majority of PA requests are ultimately approved, the value of PA requirements and their impact on patient safety should be re-evaluated.
To cite this abstract in AMA style:
Wallace Z, Harkness T, Fu X, Stone J, Choi H, Walensky R. Treatment Delays Associated with Prior Authorization for Infusible Medications: A Cohort Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/treatment-delays-associated-with-prior-authorization-for-infusible-medications-a-cohort-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-delays-associated-with-prior-authorization-for-infusible-medications-a-cohort-study/