Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The Strategy Treatments aiming at Minimal Disease Activity in PsA (STAMP) is a one-year randomized controlled trial designed to investigate the efficacy of two treat-to-target (T2T) strategies in early psoriatic arthritis (PsA). The trial aims to determine the impact of early intensive treatment on long-term outcomes in PsA. This abstract presents the 6-month primary endpoint data (ACR50).
Methods: STAMP (ISRCTN76054545) is a randomized, controlled, parallel-group, open-label, multicenter trial. Patients were eligible if they had a new diagnosis of PsA within 3 months before randomization, met the CASPAR criteria, and had a Swollen Joint Count of 66 Joints (SJC66) ≥2 at baseline. Patients were randomized to either a standard of care (SoC) arm or an early secukinumab (eSEC) arm, which used secukinumab as the first-line treatment, through block randomization per center.
Both treatment arms followed the T2T design. In the eSEC arm, treatment was initiated with Secukinumab 300mg every 4 weeks, Methotrexate (MTX) 15mg/week (oral), and a single injection of Methylprednisolone (MP) 80mg. If the target response was not achieved, treatment was escalated to a TNF inhibitor with MTX 25 mg/week, followed by a second TNF inhibitor with MTX 25mg/week if necessary. In the SoC arm, the initial treatment consisted of MTX 25mg/week and a single injection of MP 80mg. If needed, treatment was escalated to MTX 25mg/week with Sulfasalazine 2000mg/day, and subsequently to a TNF inhibitor with MTX 25mg/week.
Clinical evaluations occurred every 3 months. Therapy adjustments were made at Week 12 if there was not a 50% improvement in SJC66, and at Week 24 if MDA was not achieved. The primary endpoint was the ACR50 response, with multiple secondary efficacy endpoints (ACR20, ACR70, MDA, Resolution of enthesitis (LEI), Resolution of dactylitis, PASI75, PASI90) and health outcomes assessed.
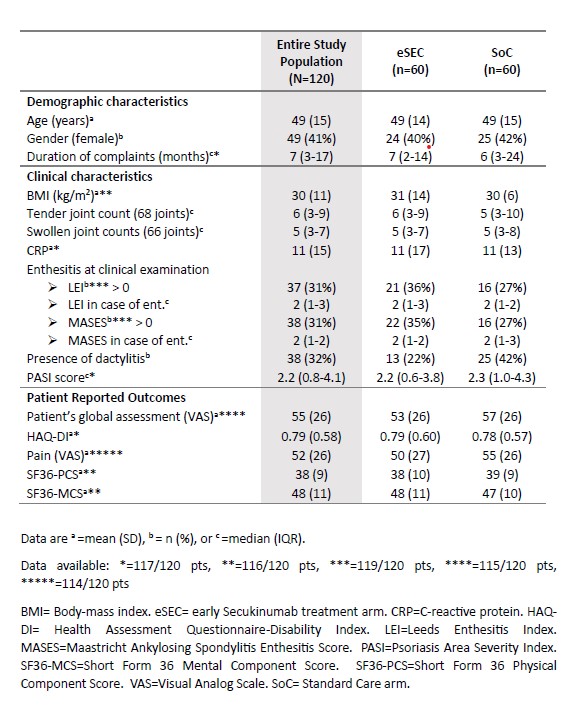
Results: A total of 120 patients were enrolled, with 60 randomized to the eSEC group and 60 to the SoC group. Baseline characteristics were well-balanced between the groups, with a mean age of 49 years and 41% female (Table 1).
By 24 weeks, the completion rates were 98.3% for the eSEC group and 83.3% for the SoC group. Most patients in the eSEC arm remained on their initial treatment, with fewer escalation of therapy compared to the SoC arm (68% vs. 50%).
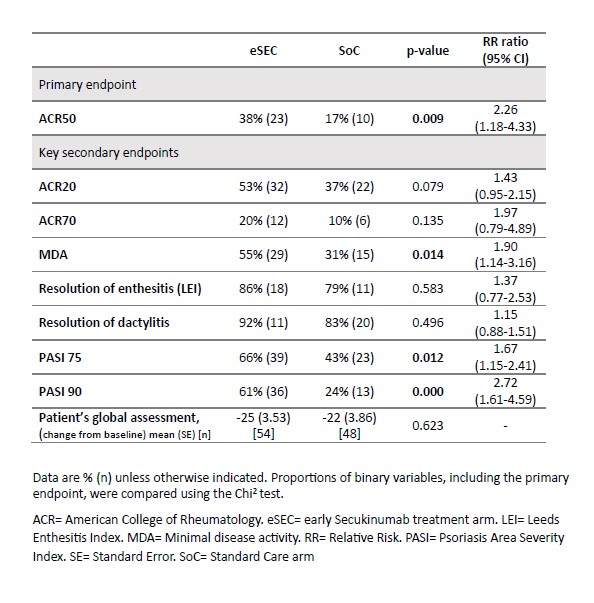
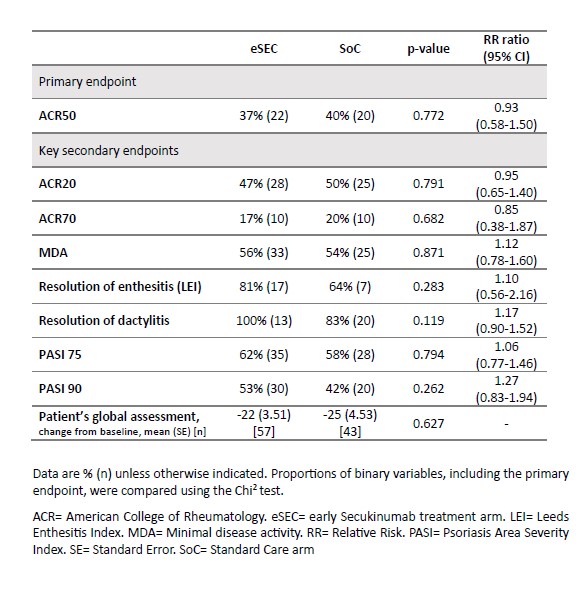
At Week 12, the primary outcome of ACR50 response was achieved by 38% of patients in the eSEC arm compared to 17% in the SoC arm (p = 0.009) (RR: 2.26, 95% CI: 1.18-4.33). Key secondary endpoints also favored the eSEC arm, with significantly higher rates of MDA, PASI75, and PASI90 (Table 2). By Week 24, ACR50 responses were comparable between the eSEC and SoC arms (37% vs. 40%, RR: 0.93, 95% CI: 0.58-1.50), and secondary endpoints showed no significant differences (Table 3).
Conclusion: In our treat-to-target study in early PsA (STAMP), the arm using early secukinumab treatment provided significant improvements in patients compared to standard care at 12 weeks. By 24 weeks, both treatment strategies demonstrated similar efficacy on primary and most secondary outcomes. Long-term follow-up will determine whether the early response to eSEC translates into lasting beneficial outcomes.
To cite this abstract in AMA style:
Koc G, Kok M, Kasiem F, Luime J, Tchetverikov I, Wilhelm - de Jong K, Korswagen L, Bijsterbosch J, Goekoop-Ruiterman Y, Baudoin P, Kok P, Bos R, Dolhain r, Vis M. Treat-to-Target Approaches in Early Psoriatic Arthritis: Early Secukinumab versus Standard Care – 12- and 24-Week Results from a Multicenter, Open-Label, Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/treat-to-target-approaches-in-early-psoriatic-arthritis-early-secukinumab-versus-standard-care-12-and-24-week-results-from-a-multicenter-open-label-randomized-controlled-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treat-to-target-approaches-in-early-psoriatic-arthritis-early-secukinumab-versus-standard-care-12-and-24-week-results-from-a-multicenter-open-label-randomized-controlled-trial/