Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: In recent years, several indices of disease activity in SLE have been proposed. The Systemic Lupus Erythematosus Disease Activity Score (SLE-DAS) showed a high correlation with other indices and greater predictive power to detect clinically significant changes compared to the SLEDAI-2K. It has 17 weighted items, with 4 continuous variables (arthritis, proteinuria, leukopenia and thrombocytopenia). The cutoff points of SLE-DAS to establish disease activity levels, scored by an online calculator, are: remission ≤2.08, 2.09≤ mild activity ≤7.64, and moderate/severe activity >7.64. For an instrument to be widely used, it must be translated and have its measurement properties validated. This study aimed to translate and adapt the SLE-DAS into Brazilian-Portuguese and evaluate measurement properties, including viability, of this new version (SLE-DAS Pt-BR).
Methods: This is a cross-sectional test-retest study. 127 patients >18 years that met SLICC and/or ACR classification criteria for SLE were included. 6 were excluded for not presenting the necessary exams to complete the score. The process of translation followed published guidelines, including forward translation, backward-translation, comittee discussion and pretesting. Reliability was tested using type 2.1 intraclass correlation coefficient (ICC2.1). Construct validity analyses followed specified hypotheses of SLE-DAS Pt-BR correlation/association with SLEDA-2K, Definitions of Remission in SLE (DORIS) and Lupus Low Disease Activity State (LLDAS), using Spearman correlation (rho), Mann Whitney, Kruskal-Wallis or Chi Square tests. Criterion validity used Physician Global Assessment (PGA) as gold standard and tested association and accuracy of SLE-DAS Pt-BR using Spearman correlation and ROC curve, respectively.
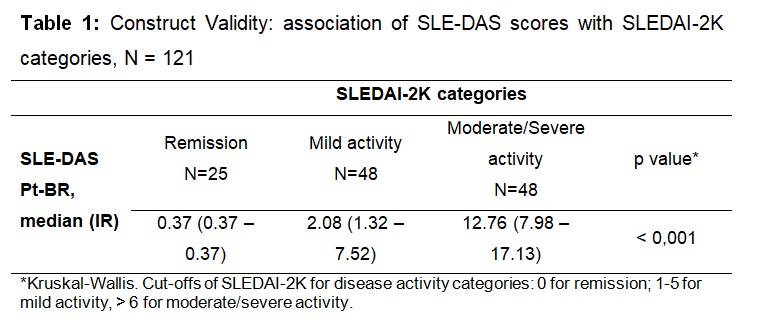
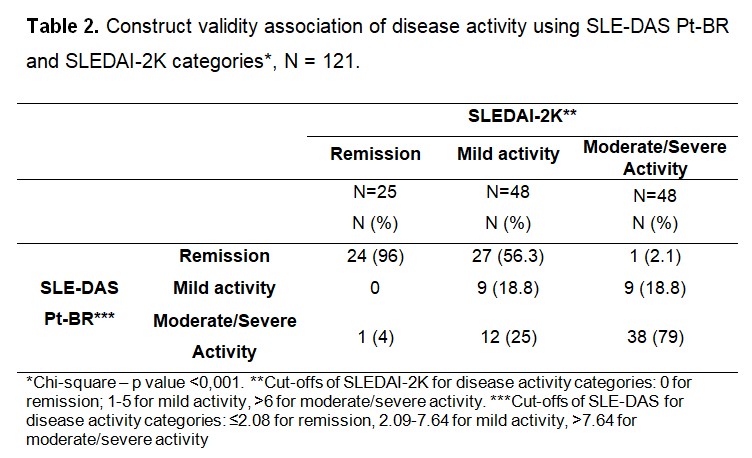
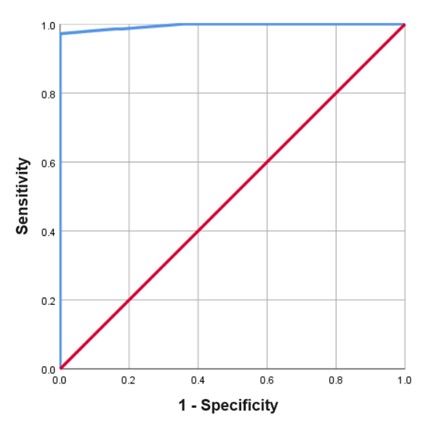
Results: The mean (SD) age was 44.3 (12.4) years, 93.4% were female sex. The majority were in remission (43%) by SLE-DAS Pt-BR. An excellent intra and inter-rater reliability was observed (ICC2.1= 0.999 and 0.945, respectively). There was a positive, strong (rho= 0.823, p< 0.001) correlation between SLE-DAS Pt-BR and SLEDAI-2K. The median of SLE-DAS Pt-BR were progressively higher from remission to moderate/severe disease activity according to SLEDAI-2K categories (p < 0.001) (Table 1). Among individuals in remission and in moderate/severe activity according to SLEDAI-2K, the majority were also in those categories according to SLE-DAS Pt-BR (Table 2). However, considering mild activity, only 18.8% were in this same SLE-DAS category (Table 2). The SLE-DAS Pt-BR scores were significantly (p < 0.001) lower in patients with remission according to DORIS and in low disease activity according to LLDAS. There was a positive, strong (rho= 0.950) correlation between the SLE-DAS Pt-BR and PGA. The cutoff point of SLE-DAS Pt-BR to define remission was 2.57, with 97.2% sensitivity and 100% specificity, and an area under the ROC curve of 99.5 (Figure 1).
Conclusion: The present study showed that the SLE-DAS Pt-BR version is reliable and viable, and has the capacity to measure SLE disease activity.
To cite this abstract in AMA style:
Mata Machado C, Lanna C, Garrido J, Moura F, Telles R. Translation, Adaptation and Validation of the Brazilian-Portuguese Systemic Lupus Erythematosus Disease Activity Score (SLE-DAS) Version: Partial Results from a Single Centre [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/translation-adaptation-and-validation-of-the-brazilian-portuguese-systemic-lupus-erythematosus-disease-activity-score-sle-das-version-partial-results-from-a-single-centre/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/translation-adaptation-and-validation-of-the-brazilian-portuguese-systemic-lupus-erythematosus-disease-activity-score-sle-das-version-partial-results-from-a-single-centre/