Session Information
Session Type: Poster Session
Session Time: 10:30AM-12:30PM
Background/Purpose: Fibromyalgia (FM) is characterized by chronic widespread pain that is often exacerbated by movement. FM is associated with enhanced central pain transmission, while transcutaneous electrical nerve stimulation (TENS) delivers electrical current through surface electrodes to counteract this central excitability. We previously showed the efficacy of TENS in reducing movement-evoked pain (MEP) and fatigue in individuals with FM in a double-blind, randomized controlled trial; however, effectiveness of TENS in a real-world setting has not previously been tested. As physical therapists are trained to use TENS for pain management, we examined if addition of TENS to outpatient physical therapy (PT) would reduce MEP using a pragmatic trial design.
Methods: The Fibromyalgia TENS in Physical Therapy Study (FM-TIPS) was a cluster-randomized pragmatic trial conducted at 28 outpatient PT clinics from 6 healthcare systems. Each clinic was randomized to TENS+PT or PT-Only. Potential participants were screened at their first PT visit and were eligible if they had been diagnosed with FM and no contraindications to TENS. Consented participants were instructed on TENS use at PT visit 2. The intervention consisted of 2 TENS devices used daily for 2 hours during physical activity that delivered a waveform with a modulating frequency of 2-125 Hz and duration between 100-180 µs at a strong but comfortable intensity through butterfly electrodes placed on the upper and lower back. Data was captured via REDCap on days (d) 1, 30, 60, 90 and 180. The primary outcome was MEP during a sit-to-stand test at d60 (randomized phase) modeled as a between-group comparison of change in MEP from d1 pre-TENS to d60 post-TENS using linear mixed-effects models. Following d60, the PT-Only group received TENS units along with virtual instructions (extension phase).
Results: We enrolled 459 participants, of whom 384 completed baseline data and were randomized to the TENS+PT (191) or PT-Only groups (193). Baseline characteristics are shown in Table 1. MEP at d60 was significantly lower (-1.1, CI= -1.58 to -0.7; Cohen’s d=0.46) in the TENS+PT compared to the PT-Only group during the randomized phase. Within-group comparisons showed TENS+PT reduced post-TENS MEP by d30 and effectiveness persisted to d180 (Figure 1), a result also seen in the PT-Only group during the extension phase. Movement-evoked fatigue and resting pain/fatigue demonstrated a similar pattern. There was a dose-response for measured TENS use, and the patient global impression of change favored TENS+PT (p=0.001) (Figure 2). There were more participants in the TENS+PT than the PT-Only group who demonstrated at least 30% reduction in MEP (41% vs 13%, p=0.0001) and resting pain (39% vs 21%, p=0.004). At d180, 81% of respondents that remained in the study found TENS helpful and 55% used TENS daily. There were no treatment-related serious adverse events and minor adverse events related to TENS were all ≤ 7.5%.
Conclusion: TENS meaningfully reduces FM pain and fatigue. Effectiveness persists for at least 180 days. Treatment effect size is comparable to other FM therapies even in this heterogeneous real-world population. TENS is a safe, inexpensive, and readily available treatment for FM.
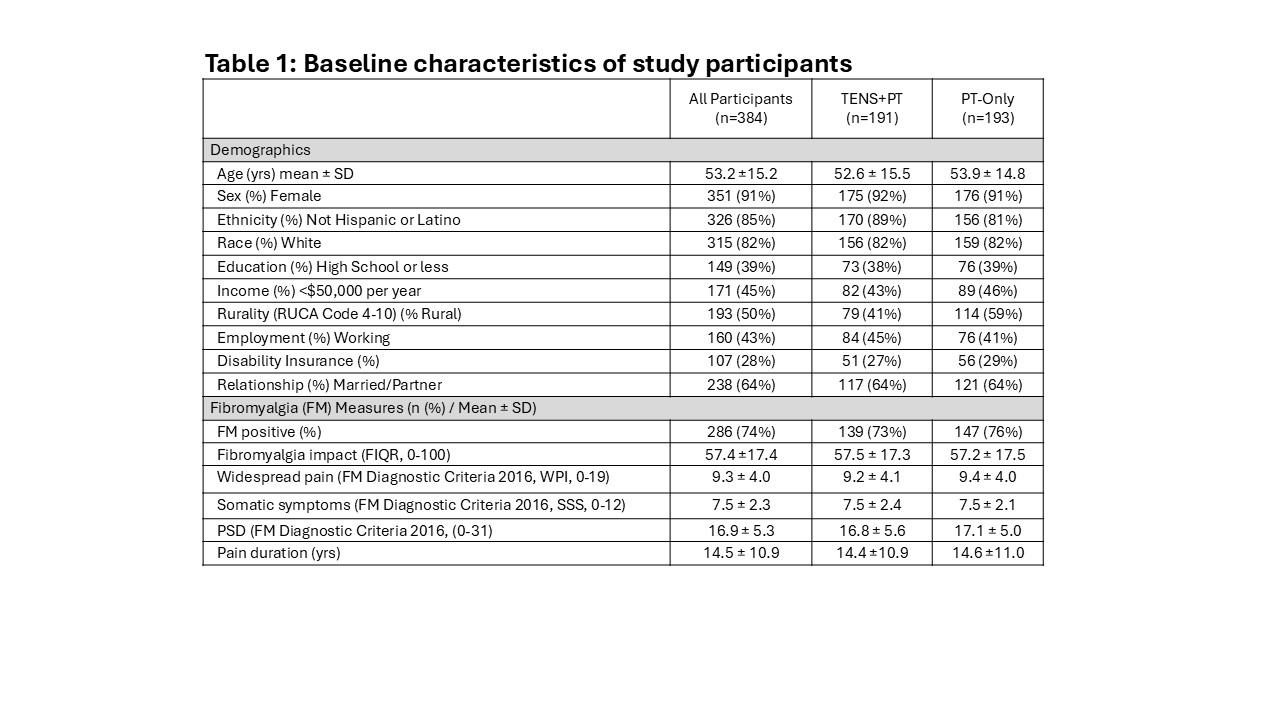 FIQR: Fibromyalgia impact questionnaire revised, WPI: Widespread pain index, SSS: Symptom severity scale, PSD: Polysymptomatic distress, PSD: Polysymptomatic distress
FIQR: Fibromyalgia impact questionnaire revised, WPI: Widespread pain index, SSS: Symptom severity scale, PSD: Polysymptomatic distress, PSD: Polysymptomatic distress
.jpg) Figure 1. Changes in A) movement-evoked pain, B) resting pain, C) movement-evoked fatigue, and D) resting fatigue before and after TENS through Day 180. Measures were assessed before (pink circles) and after 30 min application of TENS (white circles) on each day. The PT-only group tested all measures before (blue squares) and after 30 minutes of rest (white squares) on each day through day 60 (randomized phase), and before and after TENS during the extension phase on Day 90 and day 180. There were significant decreases in all measures after 30 minutes of TENS treatment for both pain and fatigue beginning on Day 30 and persisting through Day 180 for the TENS+PT group. There was no change in the measures for the PT-only group during the first 60 days, the randomized phase. However, after receiving TENS, the PT-only group showed a significant decrease 30 days later that lasted through the Day 180 test period (4 months). Data are mean and S.E.M.
Figure 1. Changes in A) movement-evoked pain, B) resting pain, C) movement-evoked fatigue, and D) resting fatigue before and after TENS through Day 180. Measures were assessed before (pink circles) and after 30 min application of TENS (white circles) on each day. The PT-only group tested all measures before (blue squares) and after 30 minutes of rest (white squares) on each day through day 60 (randomized phase), and before and after TENS during the extension phase on Day 90 and day 180. There were significant decreases in all measures after 30 minutes of TENS treatment for both pain and fatigue beginning on Day 30 and persisting through Day 180 for the TENS+PT group. There was no change in the measures for the PT-only group during the first 60 days, the randomized phase. However, after receiving TENS, the PT-only group showed a significant decrease 30 days later that lasted through the Day 180 test period (4 months). Data are mean and S.E.M.
.jpg) Figure 2. A. Per protocol analysis shows a dose-response effect for change in movement-evoked pain on Day 60 during the randomized phase. Group 1 used TENS 8x/month and a total of 900 min/mo for both the first and the second 30-day period. Group 2 used TENS 8x/month and a total of 900 min/mo for the first 30 days. Group 3 used TENS less than the minimal dose. The PT-only group did not use TENS. Groups 1 and 2 showed a greater reduction in movement-evoked pain when compared to the PT-Only group. *, p < 0.05. B. The global impression of change showed a greater number of individuals reported improvement in MEP when compared to those in the PT-only group (=16.7, p=0.001).
Figure 2. A. Per protocol analysis shows a dose-response effect for change in movement-evoked pain on Day 60 during the randomized phase. Group 1 used TENS 8x/month and a total of 900 min/mo for both the first and the second 30-day period. Group 2 used TENS 8x/month and a total of 900 min/mo for the first 30 days. Group 3 used TENS less than the minimal dose. The PT-only group did not use TENS. Groups 1 and 2 showed a greater reduction in movement-evoked pain when compared to the PT-Only group. *, p < 0.05. B. The global impression of change showed a greater number of individuals reported improvement in MEP when compared to those in the PT-only group (=16.7, p=0.001).
To cite this abstract in AMA style:
Crofford L, Dailey D, Van Gorp B, Vance C, Post A, Chimenti R, Yarasir E, Johnson E, Vance K, Zimmerman B, Jiang F, Lafontant D, Koepp M, Ecklund D, Bayman E, Sluka K. Transcutaneous Electrical Nerve Stimulation Effectively Reduces Pain in Fibromyalgia: A Pragmatic Cluster-Randomized Trial Embedded in Physical Therapy Practice [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/transcutaneous-electrical-nerve-stimulation-effectively-reduces-pain-in-fibromyalgia-a-pragmatic-cluster-randomized-trial-embedded-in-physical-therapy-practice/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/transcutaneous-electrical-nerve-stimulation-effectively-reduces-pain-in-fibromyalgia-a-pragmatic-cluster-randomized-trial-embedded-in-physical-therapy-practice/
