Session Information
Date: Saturday, November 6, 2021
Title: Abstracts: Vasculitis – Non-ANCA-Associated & Related Disorders (0502–0507)
Session Type: Abstract Session
Session Time: 4:30PM-4:45PM
Background/Purpose: Giant cell arteritis (GCA) is a chronic disease, and affected patients suffer from relapses and glucocorticoid (GC)-related toxicity. Targeted therapies are emerging with the aim of achieving better disease control and reducing GC exposure. Blocking IL-6 receptor with tocilizumab has been a major advance in the treatment of GCA. However, approximately 40% of patients treated with tocilizumab in combination with GCs experience a flare or tocilizumab-related adverse event. Blocking GM-CSF receptor a with mavrilimumab significantly reduced risk of relapse and improved sustained remission at week 26 vs placebo in a Phase 2 trial. Not all patients satisfactorily respond to any therapy, indicating heterogeneity in leading pathogenic pathways among patients. For these reasons, it is crucial to understand the specific impact of targeted therapies on vascular lesions.
In this study we investigated transcriptomic changes induced by tocilizumab or mavrilimumab in ex-vivo cultured arteries from patients with GCA.
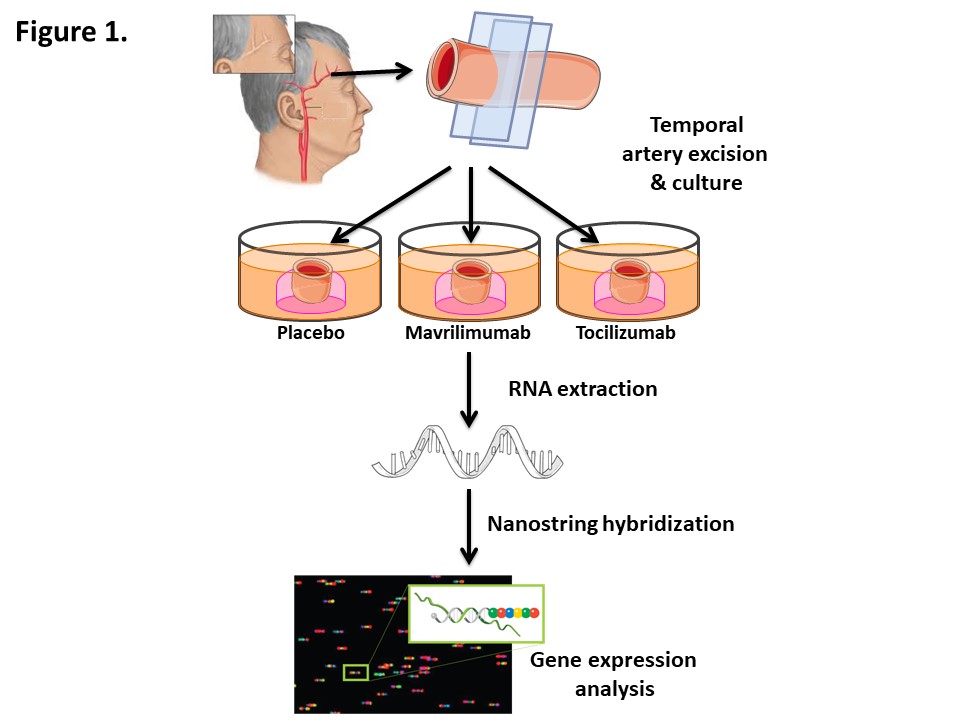
Methods: Temporal artery sections obtained for diagnostic purposes from 11 patients with histopathologically-confirmed GCA and 3 controls were cultured ex-vivo and exposed to placebo, mavrilimumab, or tocilizumab (both at 20 µg/mL) for 5 days (Fig 1). Of 11 GCA donors, 2 had received no treatment prior to biopsy, 2 had received a single prednisone (60 mg) dose, 1 had received 2 daily doses, and the remaining 6 had extended treatment; in prednisone-treated patients, mean (SEM) treatment duration was 17.9 ±8.7 days. Samples were homogenized, and total RNA was extracted with TRIzol reagent. 100 ng of RNA per sample were processed with Nanostring Inflammation gene expression assay (256 transcripts) and hybridized using nCounter Prep Station. Barcode counts from nCounter Digital Analyzer were processed with nSolver 4.0 Software. Normalised data were analyzed using R Studio 4.0.5 and IBM SPSS 22.0, and paired Wilcoxon tests were applied individually to each treatment comparison group for each analysed gene.
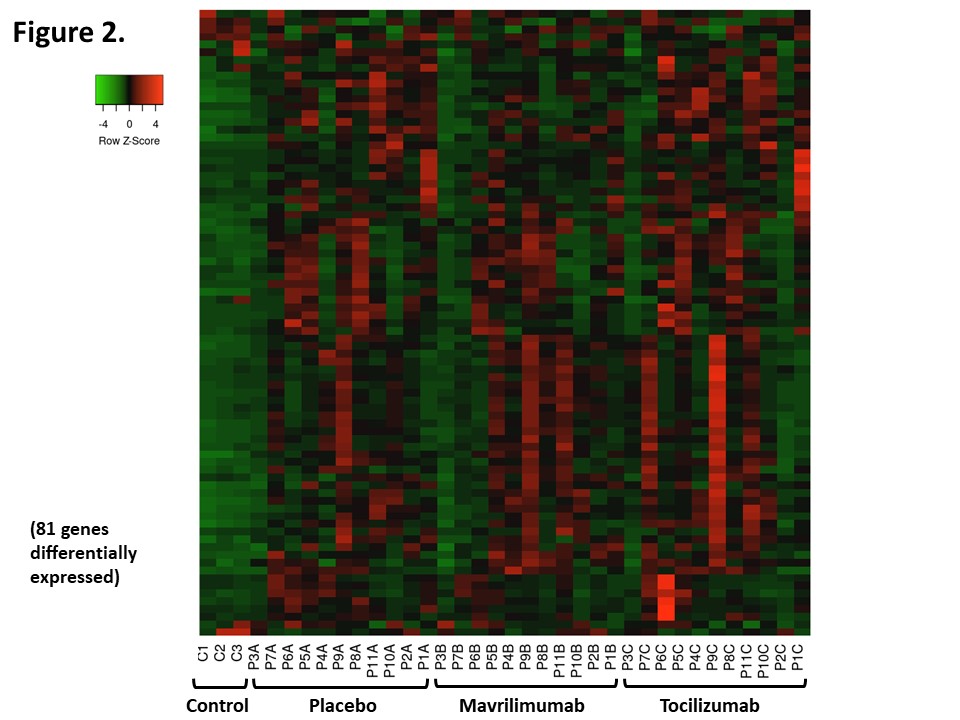
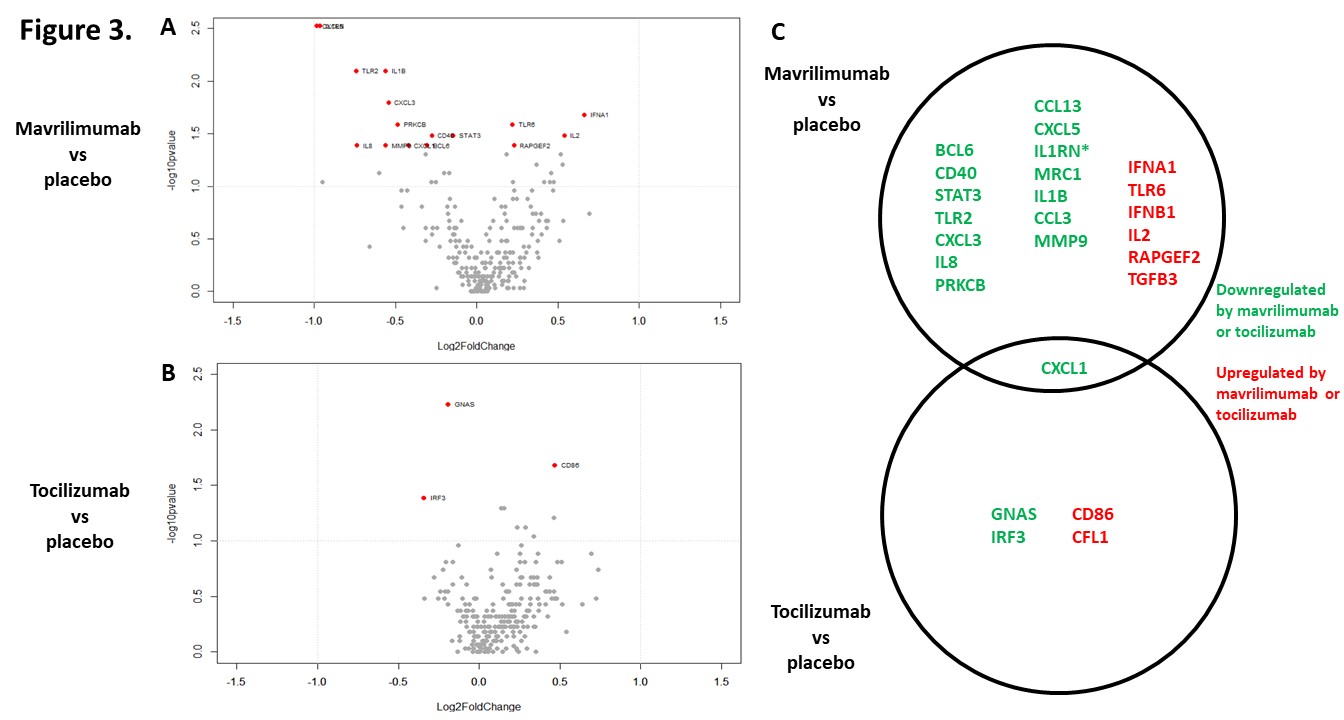
Results: 67 out of 250 transcripts were differentially expressed between arteries from GCA patients and arteries from control patients (all placebo-treated). Of those, only 9 transcripts remained significant after correction for multiple comparisons, with a false discovery rate ≤0.05. 81 transcripts were differentially expressed in at least one comparison across groups (Fig 2). 15 transcripts were lower, and 6 were higher in the mavrilimumab group vs placebo; 3 transcripts were lower, and 2 were higher in the tocilizumab group vs placebo (Fig 3A-3B). Most changes elicited between treatments were unique, but CXCL-1 was common (Fig 3C). None remained significant after correction for multiple comparisons.
Conclusion: Mavrilimumab and tocilizumab have a different transcriptomic impact on cultured arteries from patients with GCA, with some overlapping effects, although differential effects may have been attenuated by prior GC use. A better understanding of the impact of targeted therapies on vascular inflammation is needed to improve treatment options for patients with GCA.
To cite this abstract in AMA style:
Corbera- Bellalta M, Kamberovic F, Araujo F, Alba-Rovira R, Espigol-Frigolé G, Alba M, Prieto-Gonzalez S, Hernández-Rodríguez J, Pérez-Galán P, Joseph A, Paolini J, Cid M. Transcriptomic Changes Induced by Mavrilimumab versus Tocilizumab in ex-vivo Cultured Arteries from Patients with Giant-cell Arteritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/transcriptomic-changes-induced-by-mavrilimumab-versus-tocilizumab-in-ex-vivo-cultured-arteries-from-patients-with-giant-cell-arteritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/transcriptomic-changes-induced-by-mavrilimumab-versus-tocilizumab-in-ex-vivo-cultured-arteries-from-patients-with-giant-cell-arteritis/