Session Information
Session Type: Abstract Submissions (ACR)
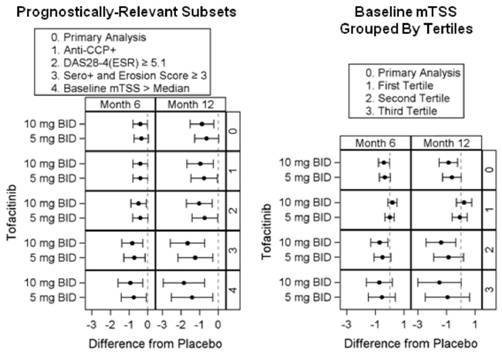
Background/Purpose: Tofacitinib is a novel, oral Janus kinase inhibitor being investigated as a targeted immunomodulator and disease-modifying therapy for RA. In the ORAL Scan trial [NCT00847613] progression in radiographic scores (mean change from baseline [BL] in modified Total Sharp Scores [mTSS] at Month 6) was a primary analysis using an Analysis of Covariance (ANCOVA). ANCOVA demonstrated statistically significant inhibition of structural damage progression for tofacitinib 10 mg but not 5 mg twice daily (BID) doses, versus placebo (PBO).1 Rank analysis of change from BL values performed as a sensitivity analysis demonstrated borderline evidence of inhibition by both doses. Mean change from BL in mTSS with PBO was <0.50 at Month 6 and also 77.7% of PBO patients showed no progression (mTSS change from BL ≤0.5). There is a trend towards less PBO progression in recent radiographic studies2 driven, in part, by the early rescue of PBO patients. In ORAL Scan, PBO treatment was only 3 months for non-responders (<20% improvement from baseline in swollen/tender joint counts, approximately 50% of patients receiving PBO) and 6 months for all others. Thus analyses focusing on prognostically-relevant characteristics of patients at particular risk of damage progression may be informative.
Methods: Literature was reviewed for factors identified as predicting higher risk of structural progression.3,4 BL data were used to subset the patients by these high-risk factors, regardless of treatment group assignment. ANCOVA was then applied to each high-risk subset.
Results: Factors reported to predict increased risk of damage progression included: anti-CCP+; BL DAS28-4(ESR) ≥5.1; both seropositive (either RF+ or anti-CCP+) and BL erosion score ≥3; and >median BL mTSS (Figure). The primary analysis of the whole data set is displayed for reference. Each of these high‑risk subsets showed maintained or increased differentiation between tofacitinib and PBO treatments. We further evaluated the predictive power of BL mTSS by tertiles, ie across three evenly divided groups irrespective of treatment assignment. In the first tertile, inhibition could not be demonstrated because of lack of progression in the PBO group. In the 2nd and 3rd tertiles, PBO progression led to large mean differences between both tofacitinib doses and PBO (Figure).
Conclusion: In prognostically relevant subsets of patients with RA, especially in patients at increased risk of radiographic progression, both tofacitinib 5 and 10 mg BID demonstrated inhibition of damage progression compared to PBO. These analyses support the conclusion of the primary analysis that tofacitinib inhibits progression of structural damage.
References: 1. van der Heijde et al. Arthritis Rheum 2011; 63: S1017-18. 2. Rahman et al. Ann Rheum Dis. 2011; 70:1631-40. 3. Smolen et al. Ann Rheum Dis. 2010; 69:964-75. 4. Singh et al. Arthritis Care Res. 2012; 64:625-39.
Dots = means; Bars = confidence intervals
Disclosure:
D. van der Heijde,
Abbott, Amgen, AstraZeneca, BMS, Centocor, Chugai, Eli-Lilly, GSK, Merck, Novartis, Otsuka, Pfizer, Roche, Sanofi-Aventis, Schering-Plough, UCB, Wyeth,
5,
Imaging Rheumatology,
4;
R. B. M. Landewé,
Pfizer Inc, Abbott, Janssen, Merck,
2,
Abbott, Amgen, Astra, BMS, Centocor, GlaxoSmithKline, Janssen, Pfizer, UCB, Vertex,
5;
D. Gruben,
Pfizer Inc.,
1,
Pfizer Inc.,
3.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tofacitinib-inhibits-radiographic-progression-in-patients-with-rheumatoid-arthritis-prone-to-develop-structural-damage-a-post-hoc-analysis-of-a-phase-3-trial/