Session Information
Date: Sunday, November 10, 2019
Title: 3S084: Systemic Sclerosis & Related Disorder – Clinical I: Therapeutics & Outcomes (863–868)
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Tofacitinib (TOFA) is a potent pan inhibitor of the Janus Kinase (JAK) family of kinases with a high degree of selectivity against JAK1 and JAK3 more than TYK2 or JAK2. This Phase I/II trial assessed the safety of TOFA 5 mg twice a day versus placebo (PLA) in diffuse cutaneous systemic sclerosis (dcSSc; clinicaltrials.gov NCT03274076).
Methods: A 6-month, 2 center, double-blind, randomized placebo-controlled trial was conducted between November 2017 and August 2018. Participants were randomized to either TOFA or matching PLA. Key inclusion criteria included dcSSc with disease duration of ≤ 60 months (defined as first non−Raynaud phenomenon) and modified Rodnan skin score (mRSS) ≥ 10 and ≤ 45 units. Background stable immunosuppressive therapies were allowed and varicella-zoster vaccine was required (unless previously received). Open label therapy was offered at Month 6. Primary outcome included the proportion of participants who experience Grade 3 or higher adverse events (AEs) using the NIH guidance: Common Terminology Criteria for Adverse Events (CTCAE v4.031) that occur at or before Month 6. Efficacy end points include mRSS, Health Assessment Questionnaire-Disability Index (HAQ-DI), patient and physician global assessments, and the ACR composite measure: Combined Response Index in Systemic Sclerosis (CRISS) 2. The current abstract provides results from the double blind phase of the trial.
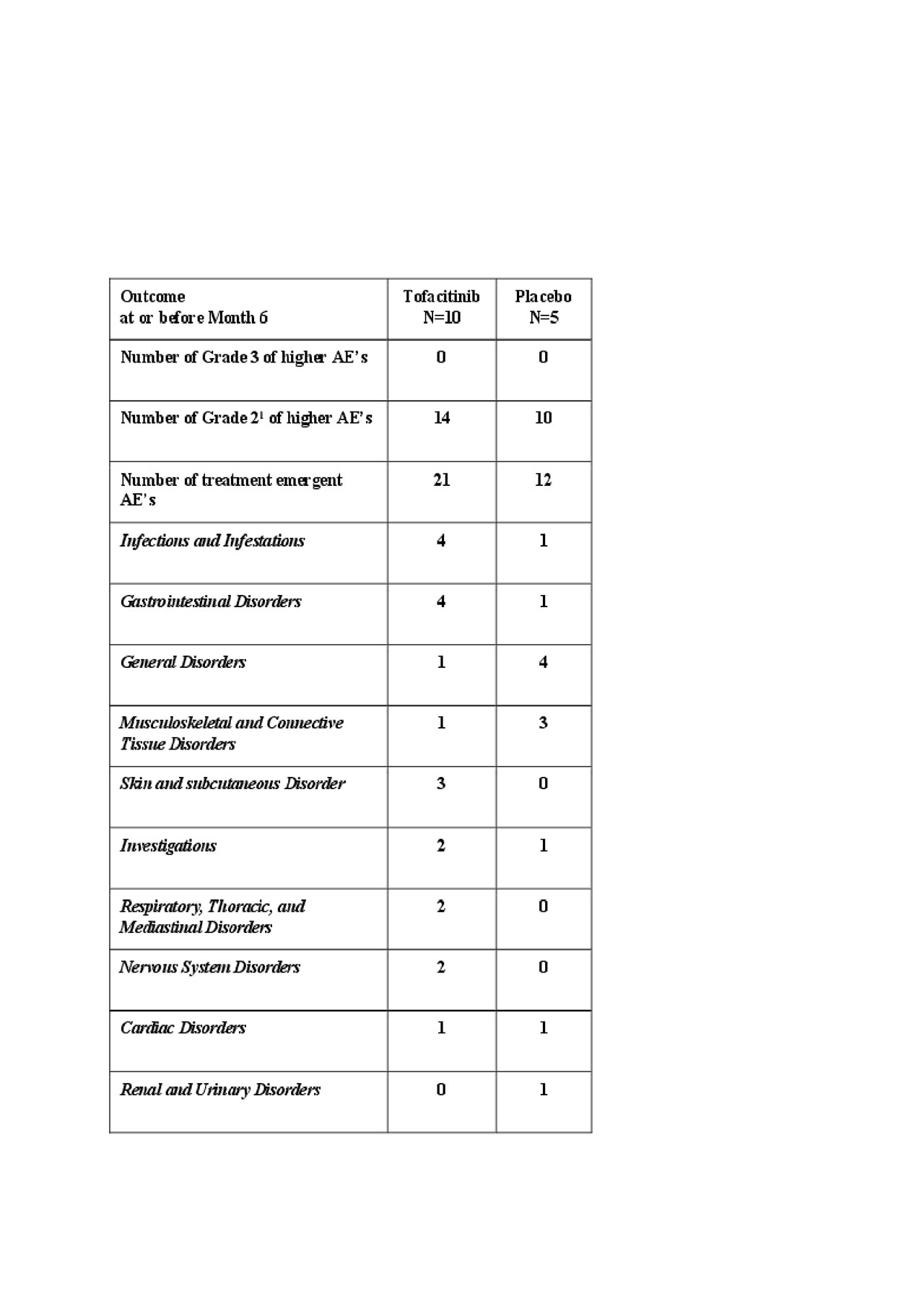
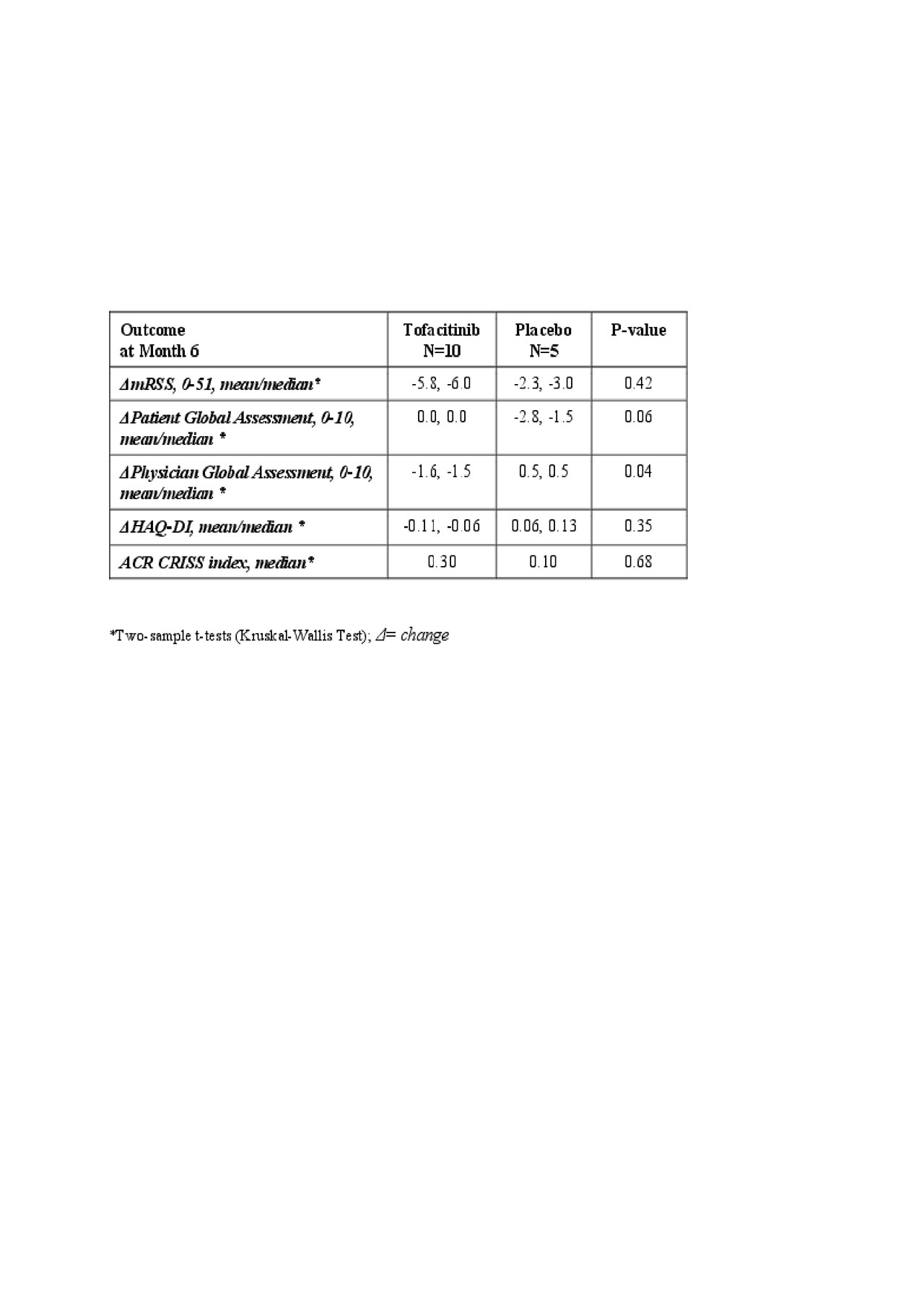
Results: 15 participants were randomized (2:1; 10 to TOFA and 5 to PLA) and formed the mITT group; 10 (100%) and 4 (80%) completed the 6-month treatment period in TOFA and PLA groups, respectively. Thirteen (13) of 15 participants were on stable daily doses of mycophenolate mofetil (mean dose=1700 mg; N=12) or weekly methotrexate (dose = 25 mg, N=1). Both the active and placebo group had 1 participant on methotrexate, leaving 8 of the TOFA participants on background mycophenolate as well as 3 in the PLA group. At baseline, mean/median age was 50.3/50.0 years, 66.7% were female, mean/median disease duration was 2.1/ 2.0 years, mean/median mRSS was 23.3/23.0, and mean/median HAQ-DI was 0.98/0.88. TOFA was well tolerated with no Grade 3 or higher AE’s before or at month 6. There were comparable AEs and AEs of special interest between treatments with no serious AEs or deaths in the trial. There were trends in efficacy favoring TOFA vs. PLA at month 6, including mRSS and ACR CRISS.
Conclusion: In participants with dcSSc, TOFA was well tolerated with manageable AEs, with no Grade 3 or higher AEs, no SAEs and there were trends in improvement of clinical outcome measures. This study supports further evaluation of TOFA in dcSSc.
Refs: 1. CTCAE v4.03
https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- Khanna D, et al. Arthritis Rheum 2016.
Acknowledgment: Dr. Khanna is funded by NIH/ NIAMS K24 AR063120 and R01 AR070470
To cite this abstract in AMA style:
Khanna D, Bush E, Nagaraja V, Koenig A, Khanna P, Young A, Moore J, Fox D, Lafyatis R. Tofacitinib in Early Diffuse Cutaneous Systemic Sclerosis— Results of Phase I/II Investigator-Initiated, Double-Blind Randomized Placebo-Controlled Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/tofacitinib-in-early-diffuse-cutaneous-systemic-sclerosis-results-of-phase-i-ii-investigator-initiated-double-blind-randomized-placebo-controlled-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tofacitinib-in-early-diffuse-cutaneous-systemic-sclerosis-results-of-phase-i-ii-investigator-initiated-double-blind-randomized-placebo-controlled-trial/