Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Tofacitinib, an oral Janus kinase inhibitor, is widely used in India to treat rheumatoid arthritis (RA) and other autoimmune diseases. Although its efficacy is well documented, its safety profile, particularly concerning tuberculosis (TB) reactivation, remains a critical concern, with an estimated TB risk of 0.2 (0.1- 0.3) per 100 patient-years and higher rates at a 10 mg twice-daily dosage. The risk of TB reactivation among tofacitinib users in India, a country endemic for TB, is under-researched.
Objectives: This retrospective cohort study aimed to assess the TB reactivation rates, other side effects, and retention rates of tofacitinib therapy.
Methods: This single tertiary referral hospital-based retrospective study analyzed patients who received tofacitinib for various rheumatic diseases between January 2021 and December 2023, with at least six months of follow-up. The baseline information included demographic data, underlying disease, tofacitinib dosage, and other concomitant treatments. Follow-up data included tuberculosis reactivation status, other side effects associated with tofacitinib therapy, dosage, duration, and retention rates.
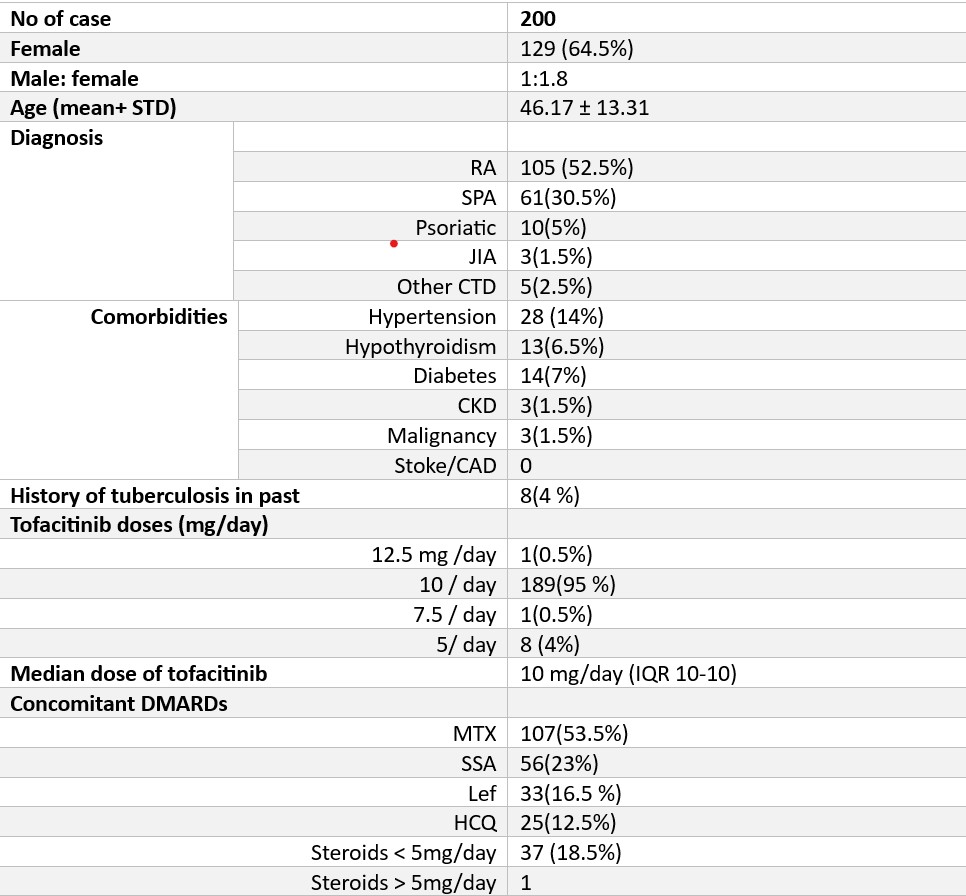
Results: We studied 200 patients with a mean age of 46.17 years (±13.31) (Table 1). The male-to-female ratio was 1:1.8. The most common diagnosis was rheumatoid arthritis (RA) in 105 patients (52.5%). A history of tuberculosis was present in 4% of the patients. Most patients (95%) received a 10 mg/day dose of tofacitinib. The concomitant medications included methotrexate (53.5%), sulfasalazine (23%), leflunomide (16.5%), and hydroxychloroquine (12.5%). Steroid use was noted in 18.5% of the patients at doses less than 5 mg/day.
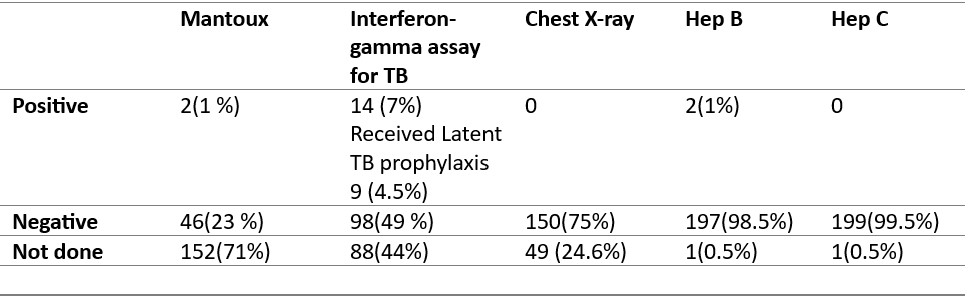
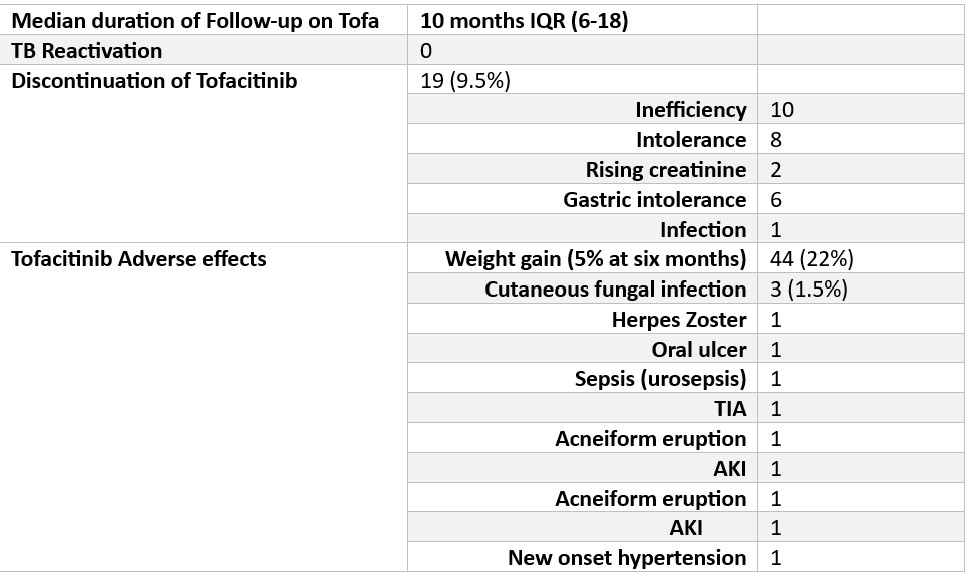
The screening methods for latent tuberculosis varied; the interferon-gamma assay (TB Gold) was conducted in 112 (56%) patients, with 14 (7%) testing positive and 9 (4.5%) receiving latent TB prophylaxis. Mantoux test was performed in 58 (29%) patients, with 2(1%) showing positive results. Chest radiography (CXR) was performed in 75% of the patients, with no positive results. (Table 2). No tuberculosis reactivation cases were observed over a median follow-up period of 10 months (IQR 6-18 months) (Table 2)
The retention rate of tofacitinib was high, with 90.5% of the patients continuing therapy. Discontinuation occurred in 9.5% of patients due to lack of efficiency (10 patients) and intolerance (8 patients).
The most common side effect was weight gain (22% of patients at six months). Other side effects include oral ulcers, infections (herpes zoster, cutaneous fungal infections, sepsis), transient ischemic attack (TIA), acneiform eruption, acute kidney injury, and new-onset hypertension. (Table 3)
Conclusion: Despite variable screening practices, at least in the short term, this study highlights the absence of tuberculosis reactivation in patients receiving tofacitinib at 10 mg/day. A high retention rate indicates tolerability, although side effects such as weight gain and infections warrant attention. The need for TB screening may be reviewed if larger data corroborates the negligible risk of reactivation of TB.
To cite this abstract in AMA style:
Kale V, yadav S, Balakrishnan C, Samant R, Swain B, Khanna S, Agrawal A, Patankar A. Tofacitinib: A Retrospective Study on Safety and Adverse Effects [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/tofacitinib-a-retrospective-study-on-safety-and-adverse-effects/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tofacitinib-a-retrospective-study-on-safety-and-adverse-effects/