Session Information
Date: Monday, November 9, 2015
Title: Rheumatoid Arthritis - Small Molecules, Biologics and Gene Therapy Poster II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Disease
remission is the current treatment goal for patients (pts) with early RA (eRA).
Initiation of an anti-TNF agent ± methotrexate (MTX) is recommended for pts
with severe eRA of poor prognosis.1 Data from 2 phase 3 clinical
trials, FUNCTION2 and AMBITION,3 have shown the efficacy
of tocilizumab (TCZ) in eRA pts, and TCZ was recently approved in the Europe
Union for the treatment of severe, active, progressive RA in MTX-naive pts.4
Data from the 2 phase 3 trials on TCZ and MTX as monotherapy in eRA pts are
presented.
Methods: In
FUNCTION, MTX-naive pts with active RA of ≤2 years’ duration received intravenous
(IV) TCZ 8 mg/kg (TCZ8) + oral MTX, IV TCZ8 monotherapy, IV TCZ 4 mg/kg + oral MTX,
or oral MTX monotherapy. In AMBITION, pts with moderate to severe RA who were
MTX naive or had not received MTX for ≥6 months received IV TCZ8 or oral
MTX as monotherapy. Twenty-four-week data are included from FUNCTION pts who
received TCZ8 or MTX monotherapy and from AMBITION pts with RA of ≤2 years’
duration (exploratory subanalysis). Key efficacy end points are remission
rates based on Disease Activity Score using 28 joints (DAS28-erythrocyte
sedimentation rate [ESR] <2.6), American College of Rheumatology (ACR)
response, and physical function assessed by Health Assessment Questionnaire–Disability
Index HAQ-DI)
at week 24.
Results:
Included
were 241 AMBITION pts (mean RA duration, 0.7 years) and 579 FUNCTION pts
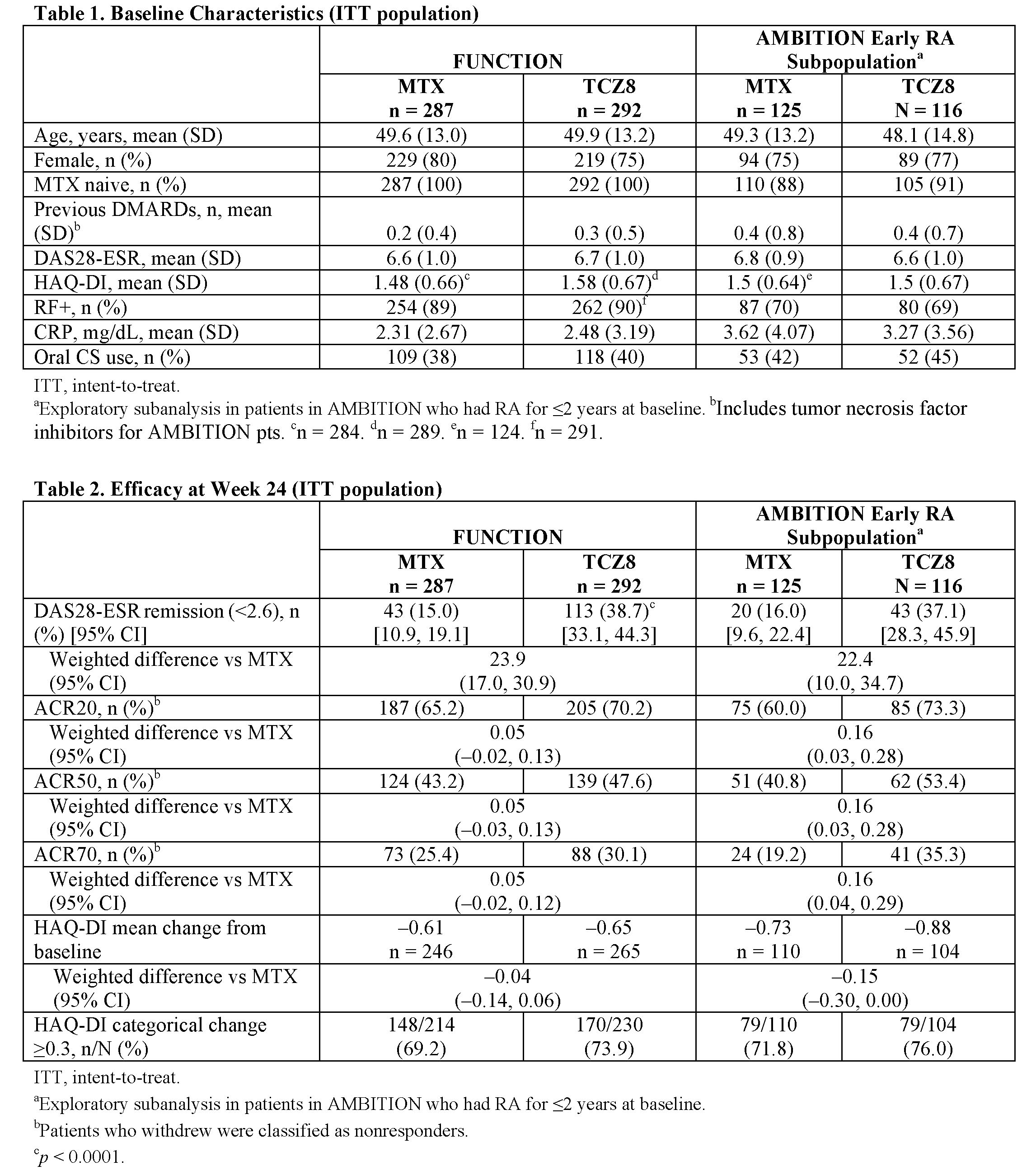
(mean RA duration, 0.4-0.5 years). Baseline
characteristics were similar across pt groups analyzed except that all FUNCTION
pts were MTX naive and had low previous rates of DMARD use, whereas 89.2% of the
AMBITION eRA subpopulation were MTX naive and had greater previous rates of DMARD
use (Table 1). Across both studies, greater proportions of pts receiving TCZ8 than
receiving MTX achieved DAS28-ESR remission (Table 2). The difference between
arms was statistically significant in FUNCTION, and the weighted difference in
response for TCZ8 compared with MTX was 22.4% in AMBITION, which is similar to
the corresponding value in FUNCTION (Table 2). In both studies,
ACR response rates were similar or numerically greater with TCZ8 than with MTX,
and change from baseline in HAQ-DI score was numerically higher with TCZ8 than with
MTX (Table 2). Safety of TCZ8 in the eRA population in
FUNCTION and AMBITION was consistent with the known safety profile of TCZ.
Conclusion:
These findings demonstrate the efficacy of IV TCZ8 as monotherapy in phase 3
clinical trials in pts with eRA who are primarily MTX naive. In these pts, TCZ8
had a safety profile consistent with that reported in patients with more
advanced disease.
References:
1. Singh JA et al. Arthritis Care Res
2012;64:625; 2. Burmester G et al. Ann Rheum Dis 2013;72(suppl 3):63; 3.
Jones G et al. Ann Rheum Dis
2010;69:88; 4. RoActemra (tocilizumab) Summary of Product Characteristics.
Welwyn Garden City, UK: Roche Registration Limited; September 2014.
To cite this abstract in AMA style:
Burmester G, Pethö-Schramm A, Keane C, Jones G. Tocilizumab Monotherapy in Early Rheumatoid Arthritis: Data from Two Phase 3 Randomized Controlled Trials [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/tocilizumab-monotherapy-in-early-rheumatoid-arthritis-data-from-two-phase-3-randomized-controlled-trials/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tocilizumab-monotherapy-in-early-rheumatoid-arthritis-data-from-two-phase-3-randomized-controlled-trials/