Session Information
Date: Monday, November 13, 2023
Title: (1554–1578) Vasculitis – Non-ANCA-Associated & Related Disorders Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Takayasu arteritis (TA) is a large vessel vasculitis predominantly affecting female of reproductive age. Tocilizumab (TCZ) is frequently used in synthetic DMARD refractory cases. Preclinical animal studies and small case reports suggest potential teratogenicity of TCZ in pregnancy. The current ACR guidance is to continue TCZ at conception but to cease upon confirmation of pregnancy. Herein we describe the use of tocilizumab throughout pregnancy in those with TA.
Methods: A total of 160 cases of TA were identified from a single center in the United Kingdom from 2016-2023. Cases limited to female who received TCZ during pregnancy were screened. Data regarding disease, medications, and pregnancy outcomes; live birth, miscarriage, fetal abnormalities, and fetal anomalies were extracted from the database.
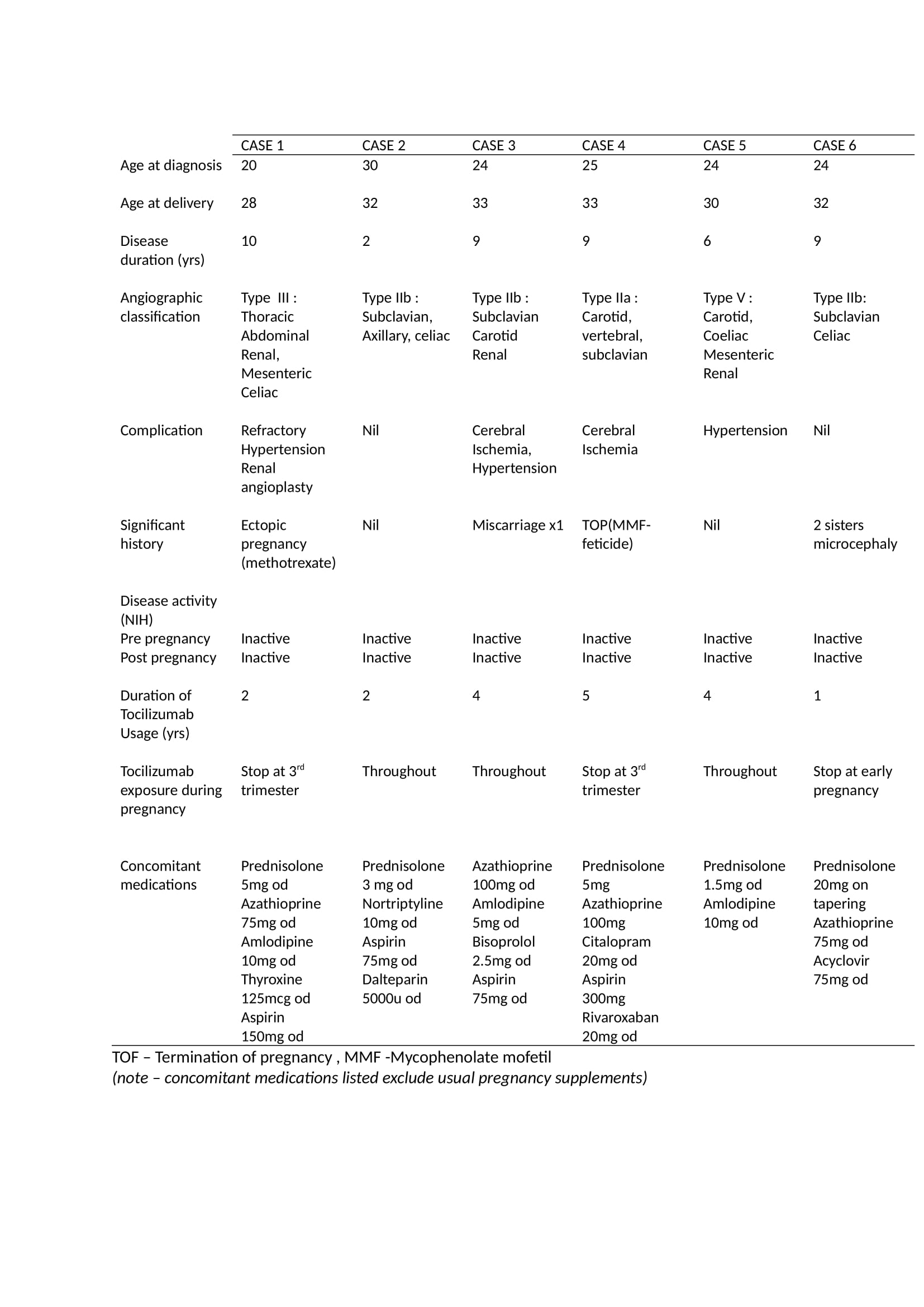
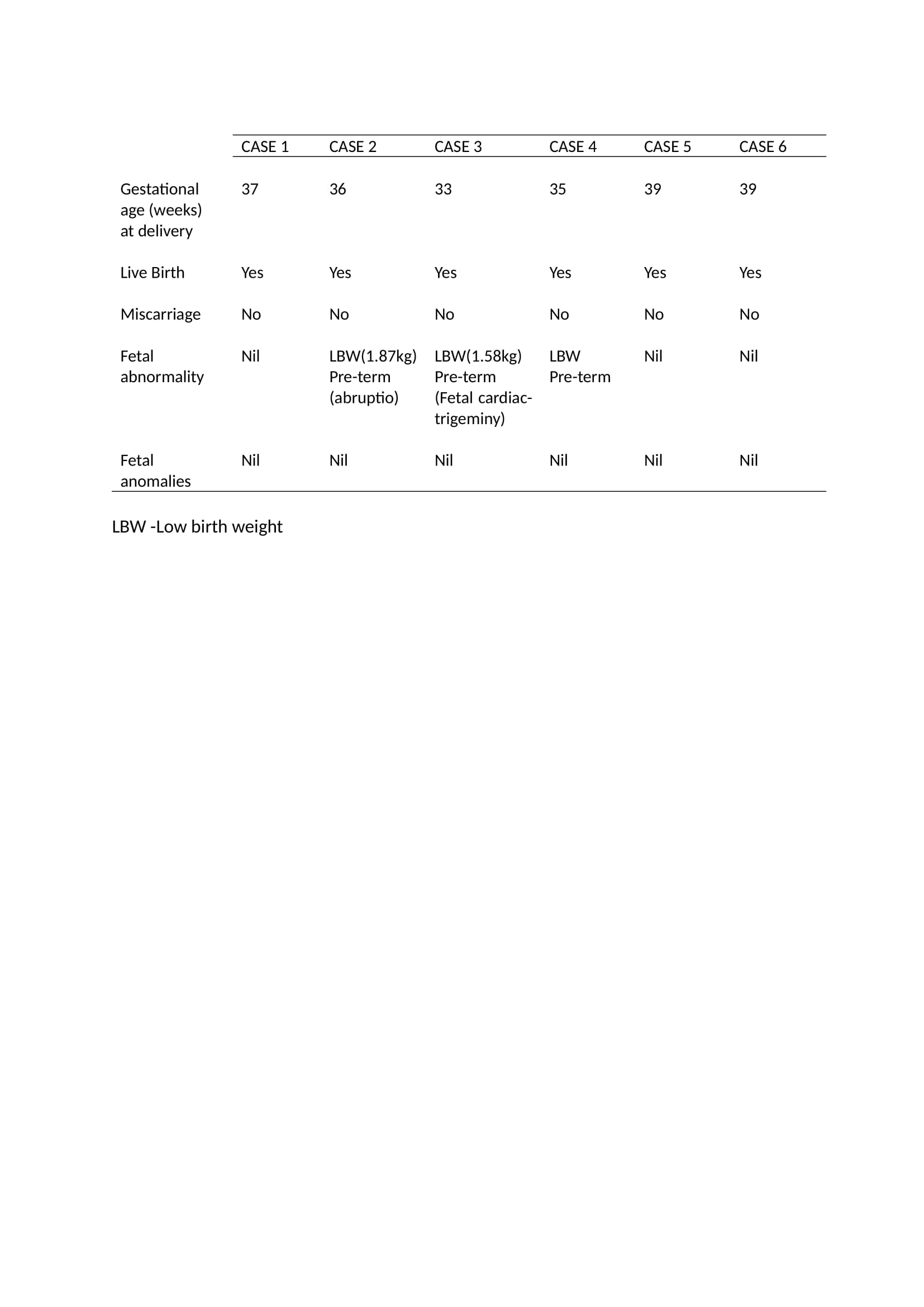
Results: Out of 160 TA patients, 45 were on TCZ between 2016-2022. Six patients were identified to have concomitant use of TCZ during pregnancy, all received Tocilizumab 162mg subcutaneously per week. The baseline characteristic and pregnancy outcomes of each case are shown in Table 1 and 2 respectively. The age at delivery range between 28 to 33 years old with the mean duration of illness of 7.5 years. In 5/6 cases the risk of flare off tocilizumab was considered higher than the potential teratogenicity. Patients and obstetric physicians were involved in the decision making. In 2 of 6 cases TCZ was stopped prior to the active transport of the monoclonal antibody across the placenta from week 30 of gestation. In 3 cases risk of flare was considered so high that TCZ was continued throughout gestation. Live vaccination of the newborn was avoided in all cases. No teratogenicity was reported following TCZ usage in TA pregnancy. However, a high proportion (50%) of pre-term deliveries and low birth weight were observed.
Conclusion: The use of TCZ in TA is well established. TA is a disease predominantly in female of childbearing age. Often TCZ cannot be stop during pregnancy. We describe six successful pregnancies exposed to tocilizumab with good maternal and neonatal outcomes
To cite this abstract in AMA style:
Zakaria N, Porter A, Maughan R, Pericleous C, Satara J, Colquhoun M, Agrawal P, Nouza j, Wilson-Morkeh H, Youngstein T, Mason J. Tocilizumab in Pregnant Takayasu Arteritis – The Use of Tocilizumab at Conception and Throughout Pregnancy in Those with Takayasu Arteritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/tocilizumab-in-pregnant-takayasu-arteritis-the-use-of-tocilizumab-at-conception-and-throughout-pregnancy-in-those-with-takayasu-arteritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tocilizumab-in-pregnant-takayasu-arteritis-the-use-of-tocilizumab-at-conception-and-throughout-pregnancy-in-those-with-takayasu-arteritis/