Session Information
Date: Saturday, November 6, 2021
Title: Abstracts: Vasculitis – Non-ANCA-Associated & Related Disorders (0502–0507)
Session Type: Abstract Session
Session Time: 4:45PM-5:00PM
Background/Purpose: PMR is the second most common inflammatory rheumatic disease of people aged 50 years or older. Glucocorticoid therapy is highly effective, but many patients require treatment for several years with significant risk of adverse events. Therefore, effective glucocorticoid sparing agents are needed. Here we investigate the efficacy and safety of tocilizumab versus placebo on background treatment with glucocorticoids in patients with new onset PMR.
Methods: In this double-blind, multi-center phase 2/3 clinical trial, we randomly assigned patients from three centers with new onset PMR in a 1:1 ratio to receive subcutaneous tocilizumab at 162mg weekly or matching placebo for 16 weeks, with 8 weeks of follow-up. All patients received oral prednisone at a starting dose of 20mg, but were rapidly tapered to nil over eleven weeks. The primary endpoint was the proportion of patients in glucocorticoid-free remission at week 16; key secondary endpoints, including time to first relapse and cumulative glucocorticoid dose at weeks 16 and 24, were evaluated. This study is registered with clinicaltrials.gov (NCT03263715).
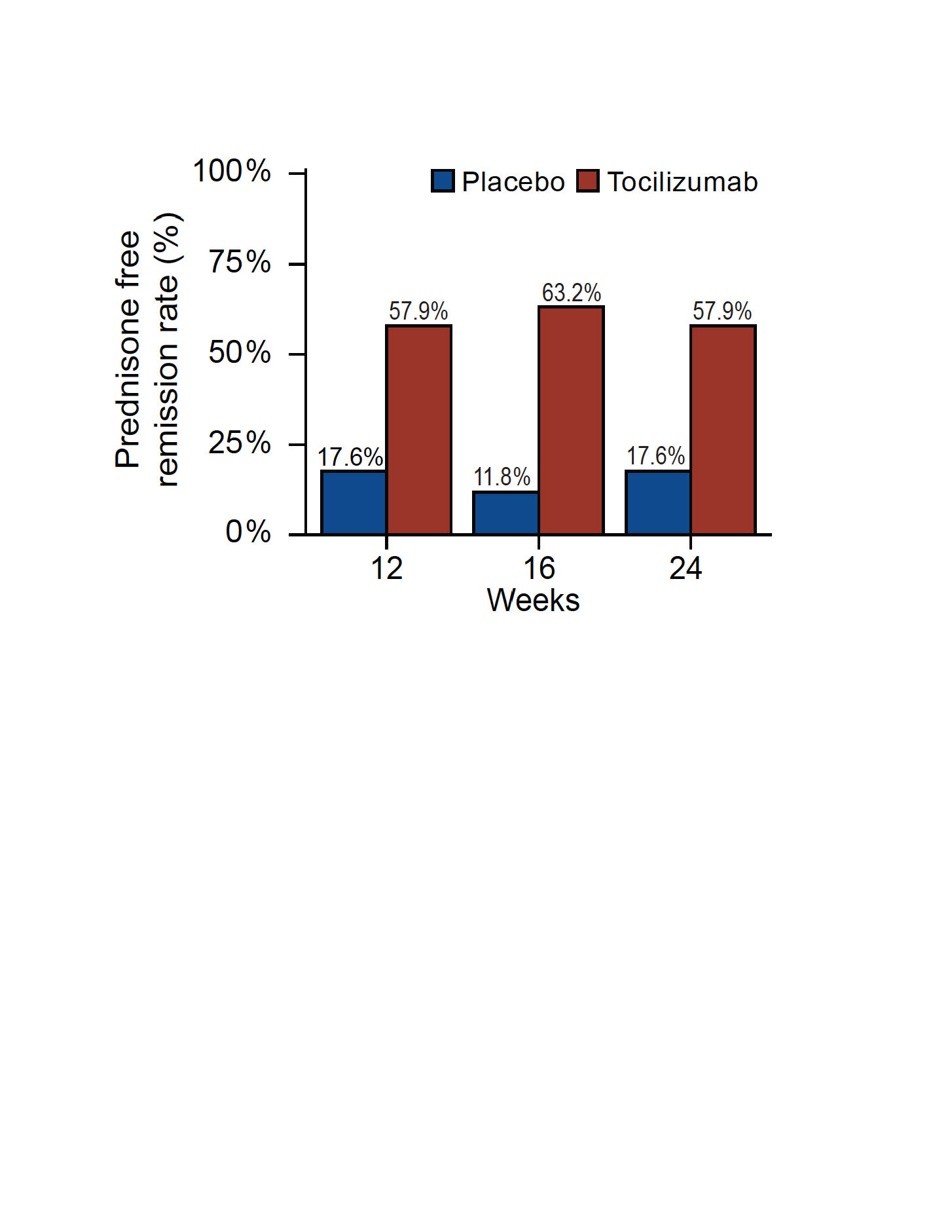
Results: In accordance with the power calculation informed by previous open studies, 36 patients were randomly assigned to tocilizumab (n=19) or placebo (n=17). Glucocorticoid-free remission at week 16 was achieved in 12/19 patients treated with tocilizumab (63.2%) vs 2/17 patients treated with placebo (11.8%), corresponding to an odds ratio of 12.9 (95 % confidence interval: 2.2 to 73.6) in favor of tocilizumab (p=0.002 by Fisher’s exact test). Mean (±SD) time to first relapse was 130±13 and 82±11 days (p=0.007), and the median (interquartile range) cumulative glucocorticoid dose was 727 (721;842) and 935 (861;1244) mg (p=0.003), respectively (Figure 1).
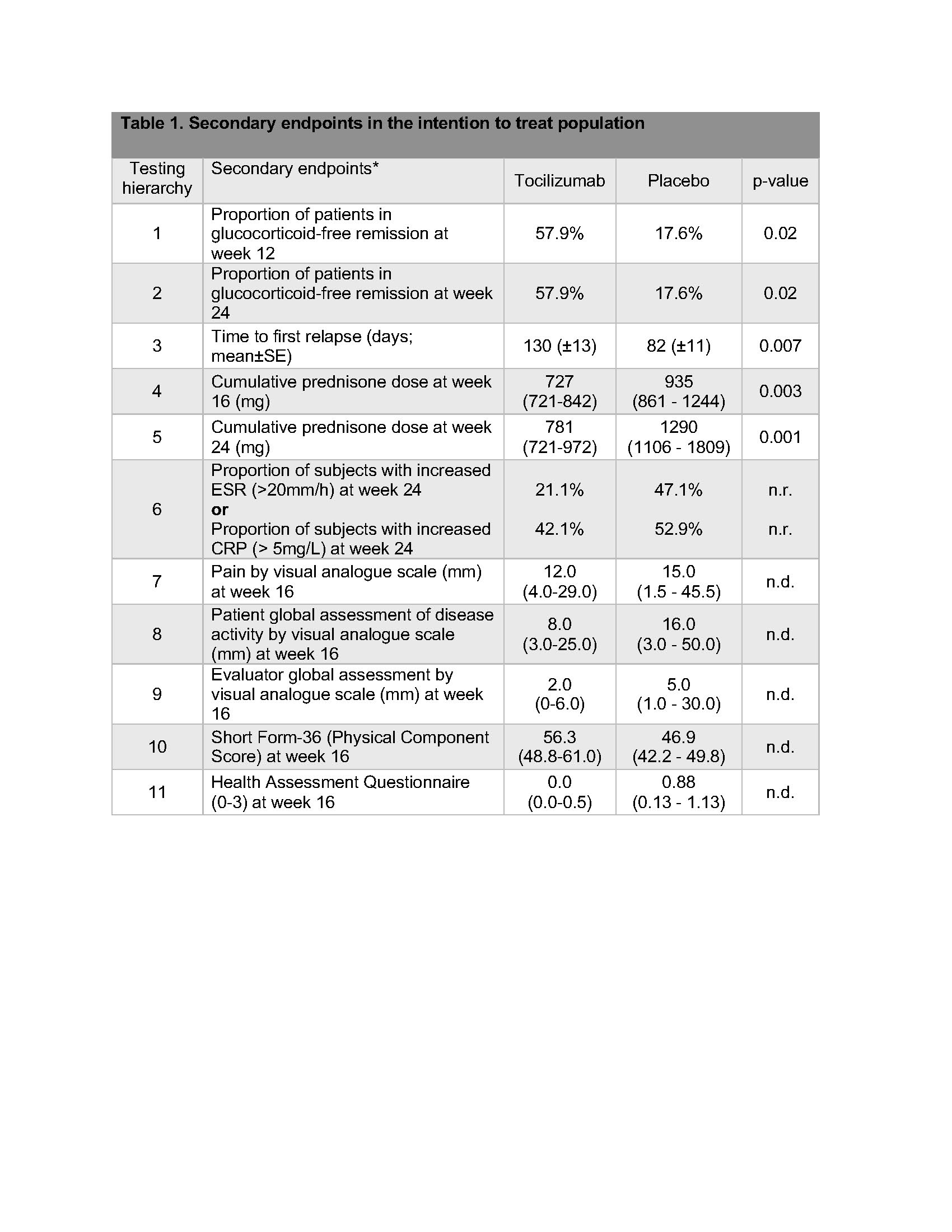
In hierarchical analyses, statistical significance was reached for many secondary outcomes including cumulative prednisone dose at week 24 (Table 1).
Serious adverse events were observed in five patients receiving placebo and in one patient receiving tocilizumab.
Conclusion: In this first randomized controlled trial of tocilizumab in PMR, patients with new onset PMR undergoing rapid glucocorticoid tapering, tocilizumab was superior to placebo regarding sustained glucocorticoid-free remission, time to relapse, and cumulative glucocorticoid dose.
n.d., not done (given the hierarchical testing rule); n.r., not reported (first non-significant value in the hierarchical testing)
To cite this abstract in AMA style:
Bonelli M, Radner H, Smolen J, Durechova M, Stieger J, Husic R, Kerschbaumer A, Dejaco C, Aletaha D. Tocilizumab in Patients with New Onset Polymyalgia Rheumatica (PMR-SPARE) – a Phase 2/3 Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/tocilizumab-in-patients-with-new-onset-polymyalgia-rheumatica-pmr-spare-a-phase-2-3-randomized-controlled-trial/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tocilizumab-in-patients-with-new-onset-polymyalgia-rheumatica-pmr-spare-a-phase-2-3-randomized-controlled-trial/