Session Information
Date: Sunday, October 26, 2025
Title: (0731–0764) Vasculitis – Non-ANCA-Associated & Related Disorders Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Tocilizumab (TCZ) is the only approved biological drug in the treatment of giant cell arteritis (GCA). However, there are no comparative studies on the efficacy of TCZ in patients with GCA with ischaemic vs. non-ischaemic manifestations. Our aim was to compare the effectiveness of TCZ in patients with GCA with ischaemic vs. non-ischaemic manifestations in clinical practice.
Methods: Multicentre observational and comparative study of 471 patients with GCA treated with TCZ. GCA was diagnosed by: i) ACR criteria, and/or ii) temporal artery biopsy, and/or iii) imaging techniques. In this case, a comparative subanalysis between patients with GCA with ischaemic manifestations (visual involvement and/or jaw claudication and/or stroke) and those with GCA with non-ischaemic manifestations was done. Remission was considered according to EULAR definitions, as the absence of signs and symptoms of GCA and the normalization of the ESR and CRP values (1).
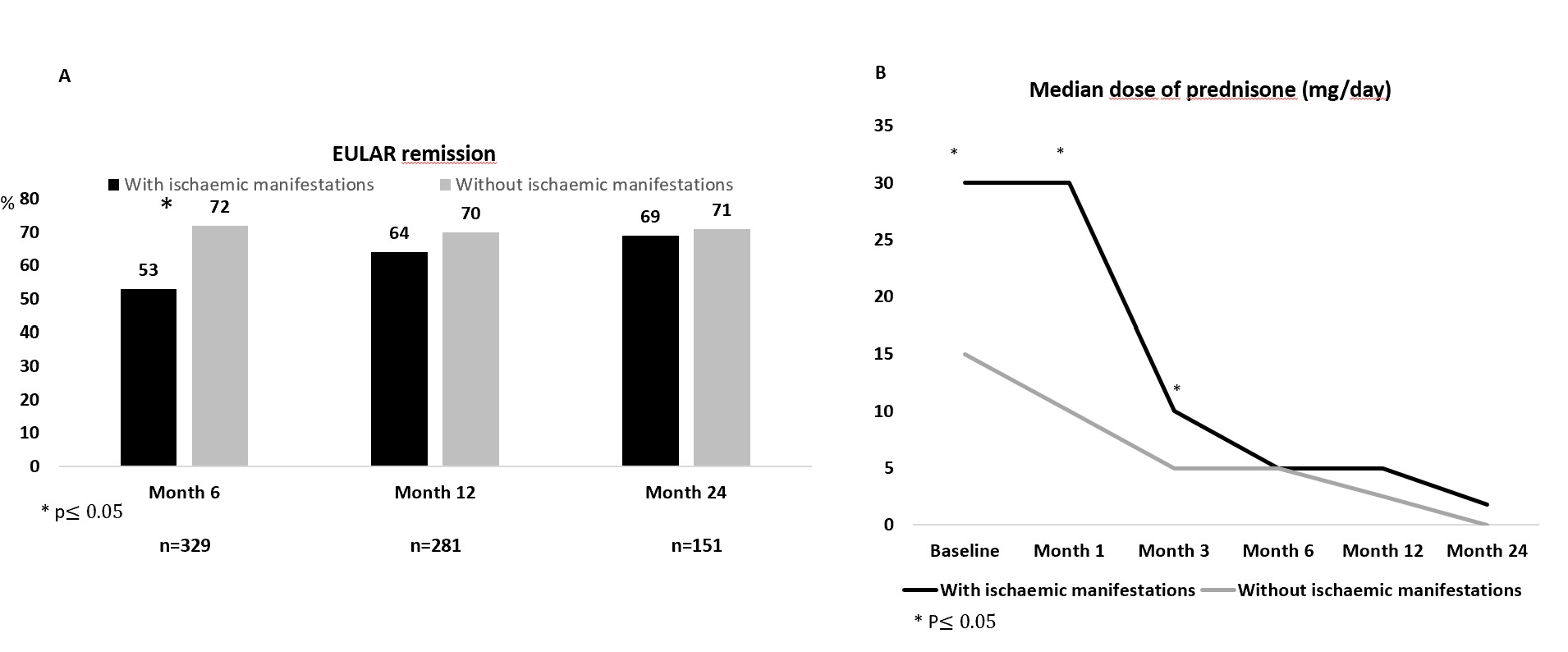
Results: The 471 patients with GCA were divided into 2 subgroups: a) GCA with ischaemic manifestations (n=162), and b) GCA with non-ischaemic manifestations (n=309) (TABLE). Patients with ischaemic manifestations were older, met ACR1990 criteria more often, had positive temporal biopsy more frequently and the time from diagnosis to onset of TCZ was shorter. The predominant phenotype in patients with ischaemic manifestations was cranial GCA and they presented more frequently hypertension and higher mean CRP and ESR values. The mean dose of prednisone was higher in this group, while concomitant use with a conventional synthetic DMARD was more frequent in the non-ischaemic group.No significant differences were observed between the two groups in terms of remission except within the 6 months of starting TCZ, which was higher in the non-ischaemic group (FIGURE). Glucocorticoid dose was higher in the GCA group with ischaemic manifestations during the first 3 months, but was similar in both groups thereafter.
Conclusion: The effectiveness of TCZ appears to be similar in patients with GCA with ischaemic and in patients with non-ischaemic manifestations, although in the latter group combined treatment was more frequent.
.jpg) TABLE. Main features of GCA patients with and without ischaemic manifestations treated with tocilizumab.
TABLE. Main features of GCA patients with and without ischaemic manifestations treated with tocilizumab.
To cite this abstract in AMA style:
Secada-Gómez C, Loricera J, Moriano C, Castañeda S, Narváez J, Aldasoro Cáceres V, Maiz O, Melero-González R, Vela Casasempere P, Romero-Yuste S, Callejas J, de Miguel E, Galíndez Agirregoikoa E, Sivera F, Ferraz Amaro I, Sánchez Martín J, Blanco R. Tocilizumab in Giant Cell Arteritis with ischemic vs non-ischemic manifestations [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/tocilizumab-in-giant-cell-arteritis-with-ischemic-vs-non-ischemic-manifestations/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tocilizumab-in-giant-cell-arteritis-with-ischemic-vs-non-ischemic-manifestations/