Session Information
Date: Monday, November 8, 2021
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Even with the use of tocilizumab (TCZ), significant glucocorticoid exposure (usually ³ 6 months) continues to be an important problem in giant cell arteritis (GCA). Therefore, we evaluated the efficacy and safety of TCZ in combination with 2 months of prednisone in a group of patients with GCA.
Methods: We conducted a prospective, single arm, open-label study of TCZ in combination with 2 months of prednisone for new-onset and relapsing GCA patients with active disease. GCA diagnosis required confirmation by temporal artery biopsy or vascular imaging. Active disease was defined as presence of cranial or polymyalgia rheumatica symptoms needing treatment within 6 weeks of baseline. All patients received TCZ 162 mg subcutaneously every week for 12 months and an 8-week prednisone taper starting between 20 mg and 60 mg daily. The primary endpoint, sustained prednisone-free remission, was defined as absence of relapse from induction of remission up to week 52 while adhering to the prednisone taper. Relapse was defined as recurrence of symptoms of GCA requiring treatment intensification, regardless of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels. Safety was also evaluated.
Results: Between 11/2018 and 11/2020 we enrolled 30 patients (mean age 74 years, 60% female, 97% Caucasian, 57% new-onset disease, 77% temporal artery biopsy-proven, 47% imaging-proven) (Table 1). The mean ESR and CRP at screening were 50.3 mm/hour and 53.2 mg/L, respectively. At this moment, 22 patients have completed the study treatment protocol and 8 patients have yet to reach week 52 (mean follow up 35 weeks). Here we report the outcomes of the 22 patients that have completed the study. The initial prednisone dose in this group was 60 mg (n = 6), 40 mg (n = 7), 30 mg (n = 4), and 20 mg (n = 5). All patients entered remission within 4 weeks of baseline. The primary endpoint was achieved by 18 (82%) patients (Table 2). The mean (SD) cumulative prednisone dose in these 18 patients was 1,037 (367) mg. After a mean period of 20.5 weeks, 4 (18%) patients relapsed (Table 2). All relapses occurred after the completion of the prednisone taper. Overall, 4 (18%) participants developed a serious adverse event (Table 2). No cases of vision loss occurred during the study.
Conclusion: Pending the final analysis of this pilot study, the results presented here suggest that 12 months of TCZ in combination with 8 weeks of prednisone could be efficacious for maintaining disease remission in patients with GCA. Confirmation of these findings in a randomized controlled trial is required.
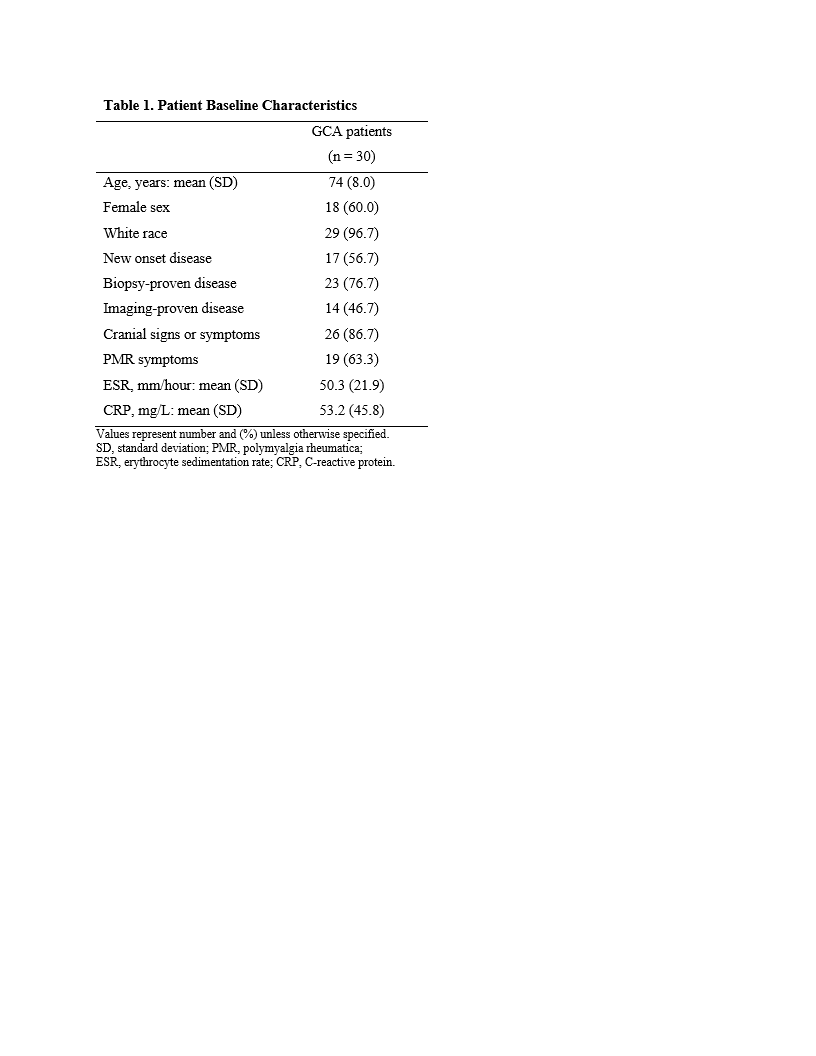 Table 1 shows patient baseline characteristics
Table 1 shows patient baseline characteristics
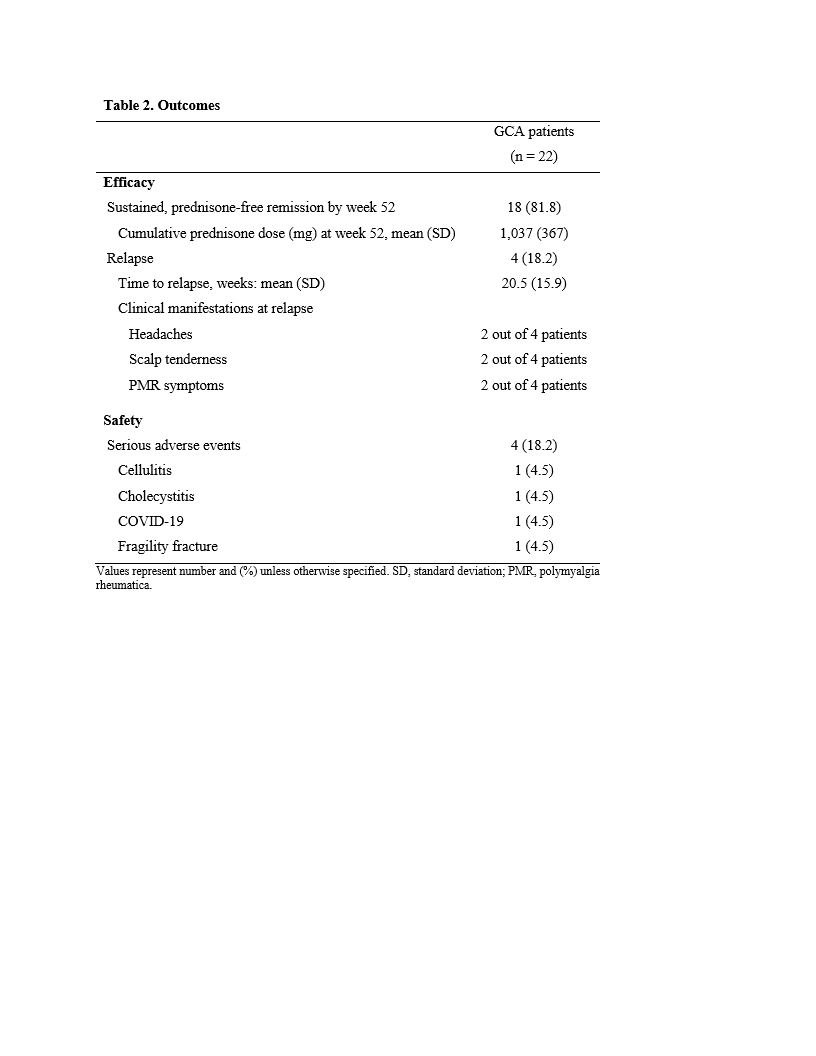 Table 2 shows outcomes for the 22 patients who have completed the treatment protocol to date
Table 2 shows outcomes for the 22 patients who have completed the treatment protocol to date
To cite this abstract in AMA style:
Matza M, Jarvie A, Fernandes A, Stone J, Unizony S. Tocilizumab in Combination with 8 Weeks of Prednisone for Giant Cell Arteritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/tocilizumab-in-combination-with-8-weeks-of-prednisone-for-giant-cell-arteritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tocilizumab-in-combination-with-8-weeks-of-prednisone-for-giant-cell-arteritis/
